Cellulose nanocrystals from renewable biomass
一种纳米晶体、纤维素的技术,应用在来自可再生生物质的纤维素纳米晶体领域,能够解决高初始投资、高运行费用、昂贵等问题,达到高产率、提高均匀性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of Cellulose Nanocrystals (CNCs) by Persulfate
[0032] The method described in this example is an environmentally friendly one-step process for the preparation of CNCs from different cellulose products. Ammonium persulfate has very high solubility in cold water (85g / 100ml), while sodium persulfate (55.6g / 100ml) and potassium persulfate (5.3g / 100ml) have lower solubility (Weast, 1983). CNCs were prepared by simply heating the cellulosic material in 1M persulfate at 60°C with vigorous stirring for 16 hours, described in more detail below using ammonium persulfate as an example. Lignocellulosic fibers such as flax and hemp are cut into short pieces (about 2-3 mm) prior to persulfate treatment. Long reaction times and persulfate concentrations above 1 M lead to excessive hydrolysis, thereby reducing the yield of CNCs.
[0033] Therefore, in one example, starting biomass material (0.1 g) was added to 10 ml of 1M ammonium persulfate solution (approxi...
Embodiment 2
[0034] Example 2: Atomic Force Microscopy (AFM) and Transmission Electron Microscopy (TEM)
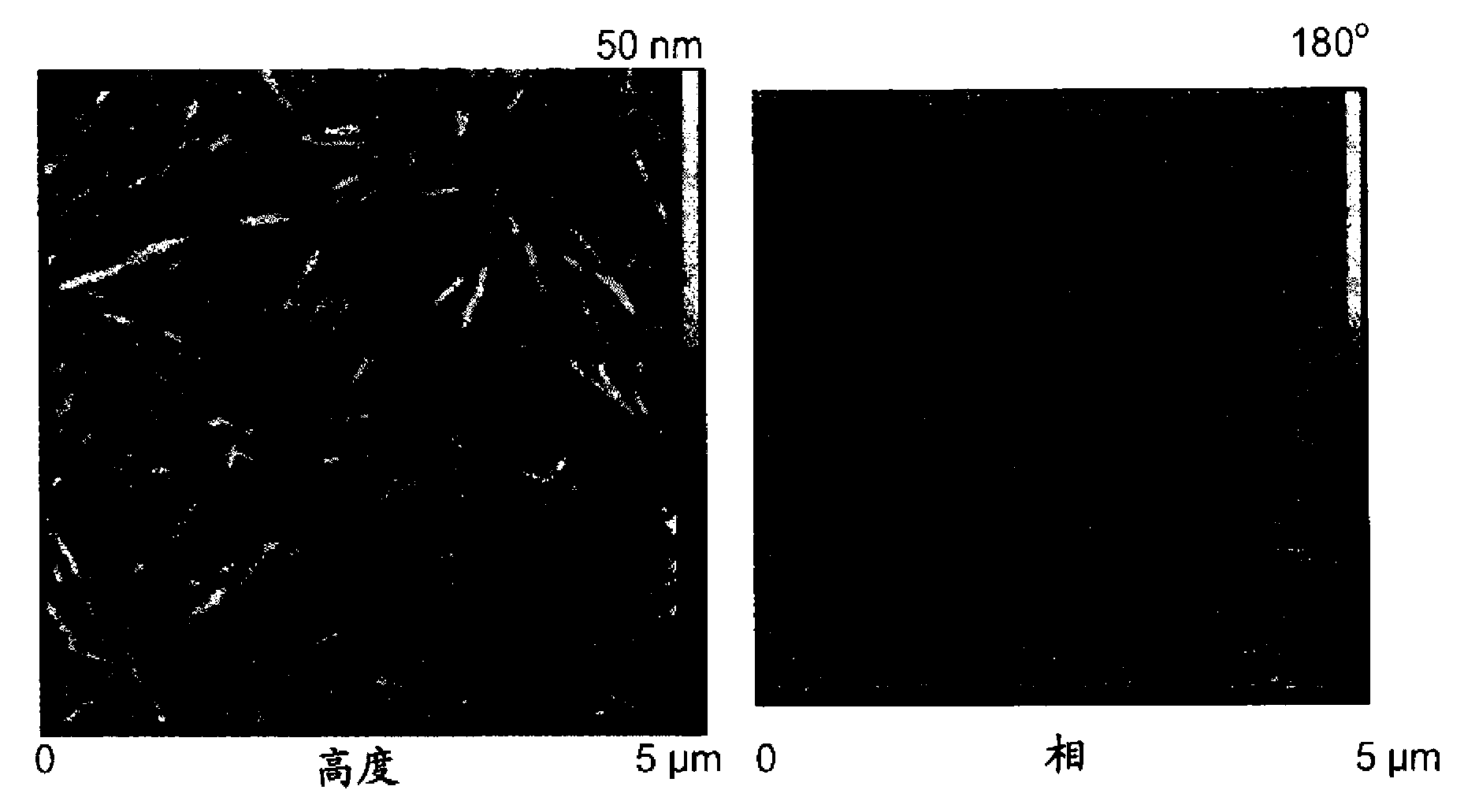
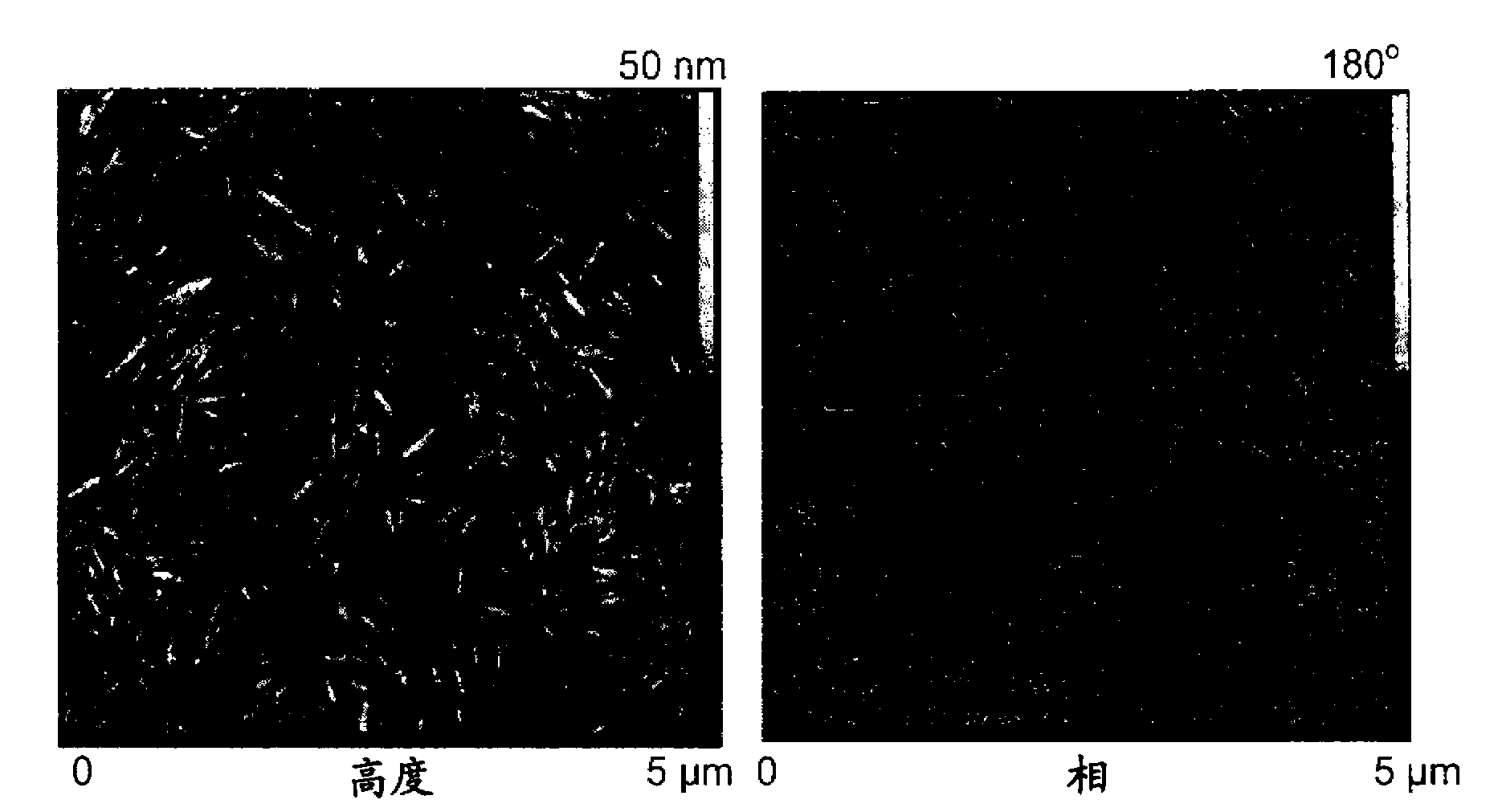
[0035] CNCs prepared using ammonium persulfate were sonicated, and atomic force microscopy (AFM) micrographs of the CNCs thus obtained were obtained using a Nanoscope TM IV (Digital Instruments, Veeco, Santa Barbara, Canada) was obtained by operating in tapping mode with a silicon tip. Particle Analysis of AFM Micrographs Using Scion TM images (http: / / www.scioncorp.com / pages / scion_image_windows.htm).
[0036] TEM micrographs were obtained by a Hitachi transmission electron microscope (TEM) at 60 kV (Model H-7500, Tokyo, Japan). TEMs were obtained as follows. A small amount of CNCs was suspended in methanol and the material was dispersed by sonication. 20 [mu]L droplets of the well-dispersed suspension were then dried on polyvinyl formal and vinyl chloride polyvinyl alcohol terpolymer (Formvar)-carbon coated grids and analyzed. Low-voltage transmission electron microscopy (LVTEM) m...
Embodiment 3
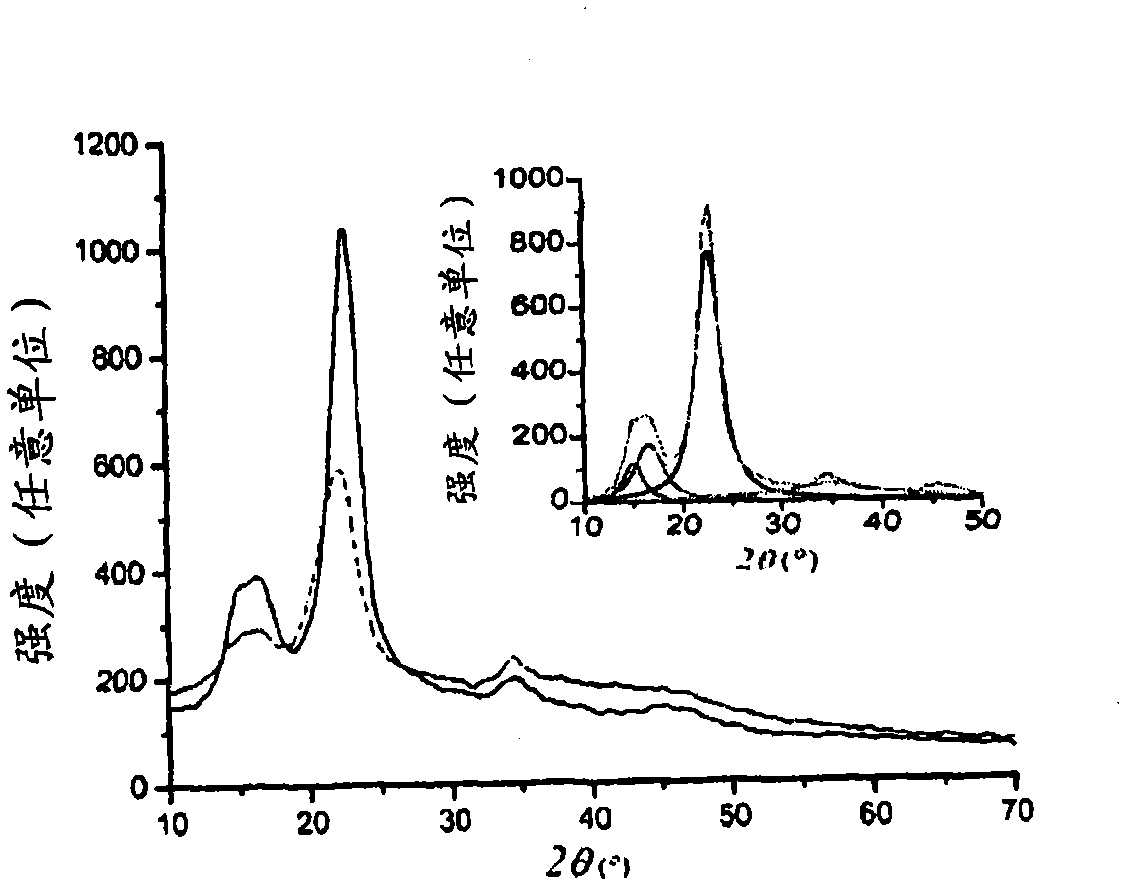
[0046] Example 3: Scanning Electron Microscope (SEM)
[0047] SEM analysis was performed by a Hitachi S2600N scanning electron microscope at 2.8 kV. SEM shows the morphological changes of the ammonium persulfate-treated fiber surface, indicating the destruction of the amorphous regions. In contrast, when the fibers were heated without ammonium persulfate, they remained intact. Ammonium persulfate enables the in situ preparation of clean CNCs by dissolving lignin, hemicellulose, pectin, and other plant components. When a solution containing persulfate is heated, free radicals (S 2 o 8 2- + heating → 2SO 4 ·- ) (Hsu et al., 2002). Therefore, persulfates are often used as initiators for emulsion polymerization in the preparation of polymers and synthetic rubbers. Furthermore, under the acidic conditions (pH 1.0) used in this study, hydrogen peroxide (S 2 o 8 2- +2H 2 O→HSO 4 - +H 2 o 2 ) (Edgar and Gray, 2003; Stiernstedt et al., 2006). Collectively, the free rad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com