Lithium-air battery
A lithium-air battery and current collector technology, which is applied to battery electrodes, circuits, electrical components, etc., can solve problems such as stability limitations, explosive reactions, and loss of activity, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] In a solution in which 28 mmol of resorcinol (Aldrich) and 120 mmol of formaldehyde (37% aqueous solution, Aldrich) were mixed, sodium carbonate and resorcin were added and mixed in a molar ratio of 45:100. The mixed solution was mixed at 75° C. for 1 hour, and then the mixture formed into a gel state was aged at room temperature for 24 hours. Afterwards, the aged mixture was washed with water and ethanol to remove sodium carbonate. The structure thus obtained was soaked in a solution of tributylphenyltin (Aldrich) for one day, and then heat-treated at 700° C. for 2 hours in an Ar atmosphere to prepare a Sn—C composite material.
[0072] The prepared Sn-C composite powder, polyvinylidene fluoride (PVDF) and carbon black (superP) were mixed in a weight ratio of 80:10:10, and dispersed in N-methyl-2-pyrrolidone to prepare Negative electrode active material layer composition. After casting the above-mentioned negative electrode active material layer composition on the co...
Embodiment 2
[0076] Mix 100 nm-sized Si powder and 5 μm-sized natural graphite powder at a weight ratio of 30:70, and mix them in a tetrahydrofuran solution. After mixing 33 parts by weight of pitch based on 100 parts by weight of the above mixed solution, ball milling was performed for 12 hours. The above mixed solution was dried in a vacuum oven at 100° C. for 6 hours, and then heat-treated in an Ar atmosphere at 1000° C. for 5 hours, thereby preparing a Si—C composite material.
[0077] The prepared Sn-C composite material powder, carbon black (super P), carboxymethyl cellulose and styrene-butadiene rubber were mixed in water at a weight ratio of 85:5:3.3:6.7 to prepare the negative electrode active material layer combination things. After casting the above-mentioned negative electrode active material layer composition on the copper foil, the cast electrode was dried in an oven at 100° C. for 2 hours, and then vacuum dried for more than 12 hours, thereby preparing a negative electrode....
experiment example 1
[0086] [Experimental Example 1: Evaluation of Electrochemical Characteristics of Li-air Battery]
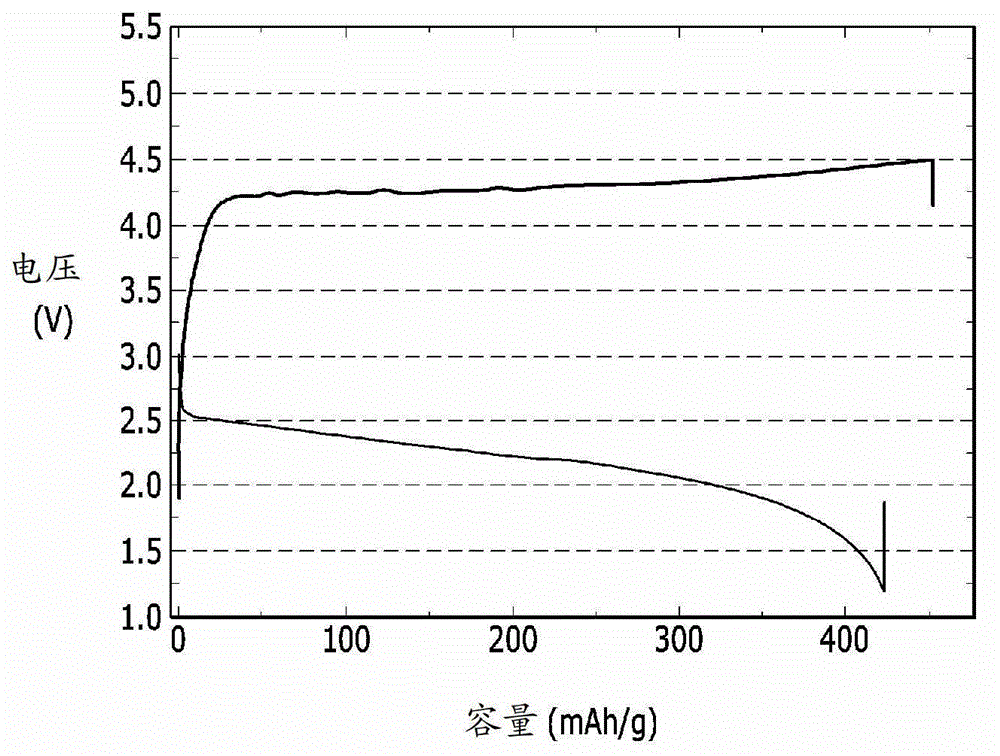
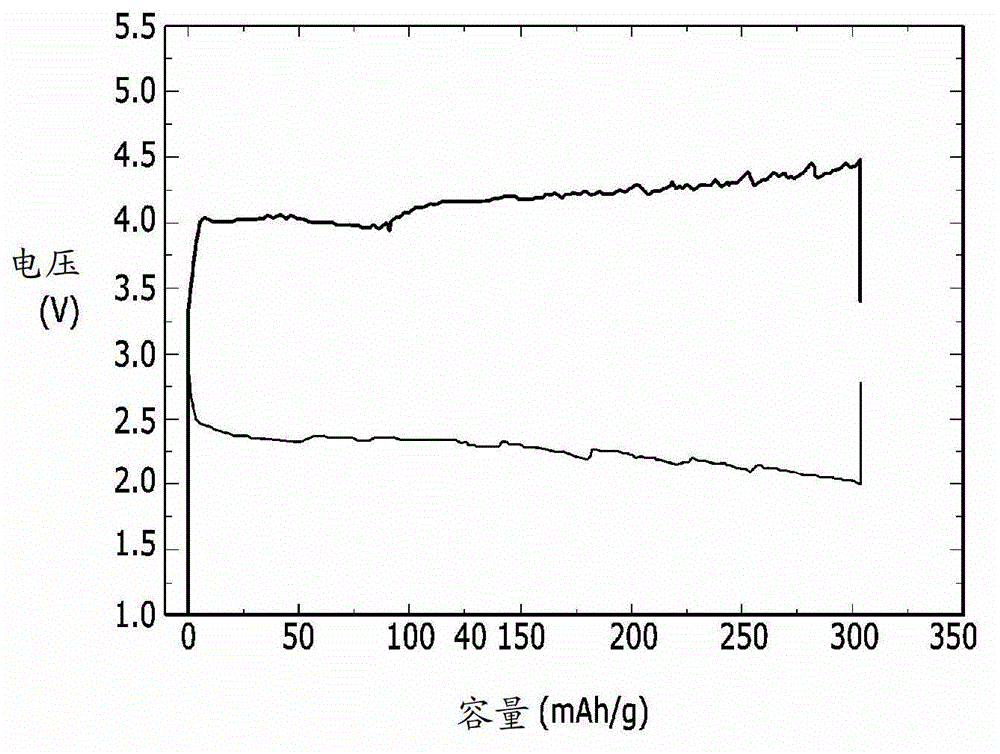
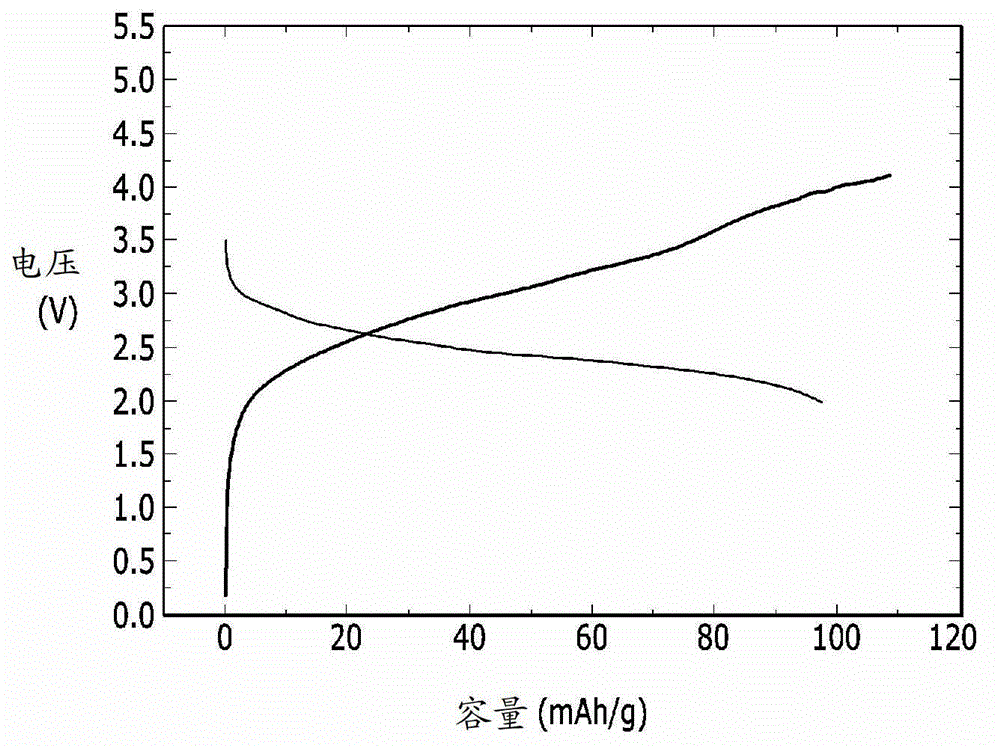
[0087] In order to evaluate the electrochemical characteristics of the lithium-air battery, the charge-discharge performance of the lithium-air battery of the above-made examples 1 and 2, comparative examples 1 and 2 was evaluated, and the results are shown in Figure 1-4 middle.
[0088] The lithium-air battery of Example 1 was placed in a chamber filled with oxygen, and then charged and discharged once at 1.2-4.5V with a current of 10mA / g. In addition, the lithium-air battery of Example 2 was charged and discharged once at a current of 5 mA / g at 2.0 to 4.5 V. In addition, the lithium-air batteries of Comparative Examples 1 and 2 were charged and discharged once at a current of 10 mA / g at 2.0 to 4.1 V.
[0089] figure 1 is a graph showing the charge and discharge performance of the lithium-air battery of Example 1, figure 2 is a graph showing the charge and discharge perfor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com