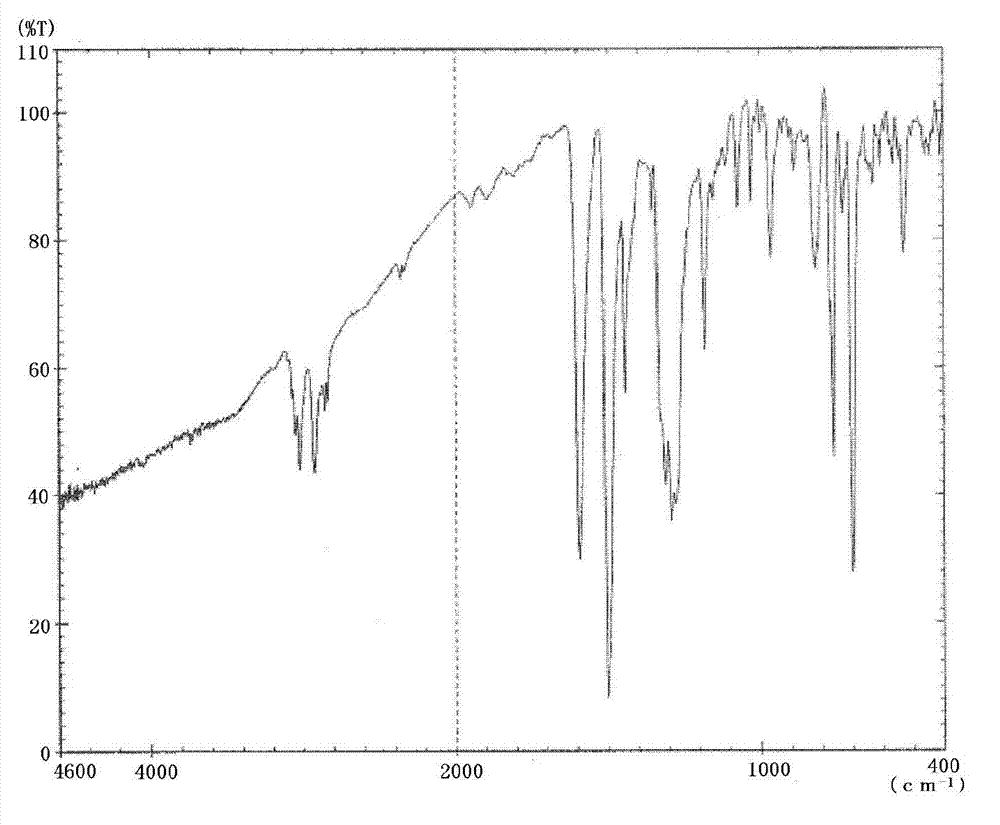
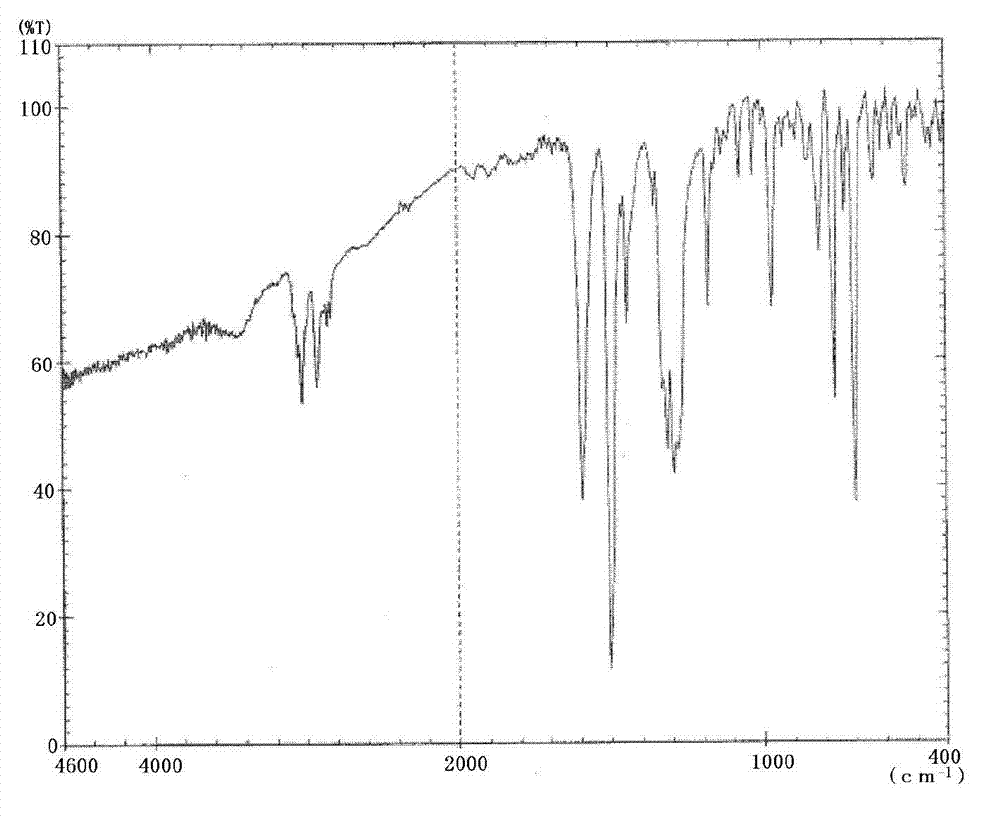
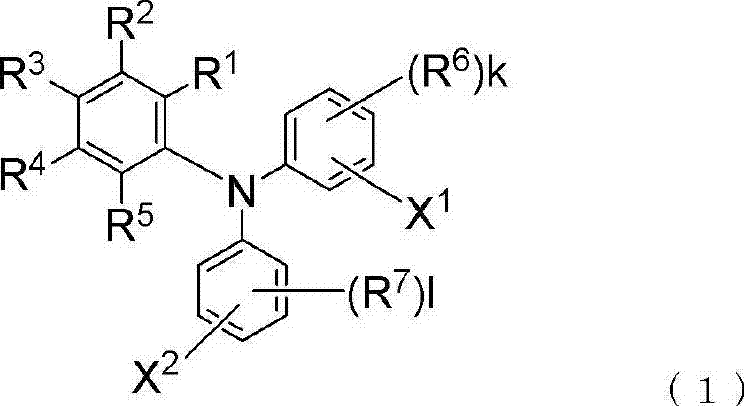
Triphenylamine derivative
A derivative, triphenylamine technology, applied in the field of triphenylamine derivatives, can solve problems such as image defects and dielectric breakdown, and achieve the effects of less sensitivity reduction, low residual potential, and excellent durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0190]
[0191] The triphenylamine derivative of the present invention described above can be synthesized by using a triphenylamine compound represented by the following general formula (4) as a starting material.
[0192]
[0193] In the above general formula (4), R 1 to R 7 , k and l are as defined in general formula (1).
[0194] The aforementioned triphenylamine compound is known, for example, as disclosed in JP-A-9-292723 (Patent Document 10). The triphenylamine derivative represented by the above-mentioned general formula (1) of the present invention passes the group X 1 Introducing a triphenylamine compound followed by introducing the group X into it 2 to prepare.
[0195] (group X 1 introduction)
[0196] In order to group the group X 1 Introduce in the triphenylamine compound of general formula (4), at first, carbonyl (formyl and ketone group) is introduced into the benzene ring that is bonded to the N atom of above-mentioned compound, thereby synthesizes ...
Embodiment
[0374] The present invention will now be specifically described by means of examples, by which, however, the present invention is by no means limited.
Synthetic example 1
[0375] [Synthesis Example 1 (Synthesis of Example Compound 1)]
[0376] A compound represented by the following structural formula (16) (see Patent Document 10) is provided as a raw material.
[0377]
[0378] 15 g of the above compound, 4.5 g of N,N-dimethylformamide, and 8 g of toluene were added to the reaction vessel, and 9 g of phosphoryl trichloride was further added dropwise thereto. The mixture was heated and stirred at 80° C. for 3 hours, and after being left to cool, 8 g of water was added dropwise thereto while cooling, followed by adding sodium carbonate to make the reaction solution alkaline.
[0379] Next, after heating at 60° C. for 3 hours, the reaction product was extracted with toluene. After washing with water and then with saturated brine, the solution was dried by using magnesium sulfate. Thereafter, the solvent was distilled off, thereby obtaining 14.4 g of a yellow solid formyl compound represented by the following structural formula (17).
[0380]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com