Method for producing industrial-grade potassium dihydrogen phosphate (KH2PO4) by wet method purified phosphoric acid and potassium sulfate
A technology of grade potassium dihydrogen phosphate and potassium dihydrogen phosphate, which is applied in the field of phosphorus chemical industry, can solve the problems of high product price, economic inability to pass the customs, high price, etc., achieve easy control and industrial application, avoid wrapping phenomenon, production Simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
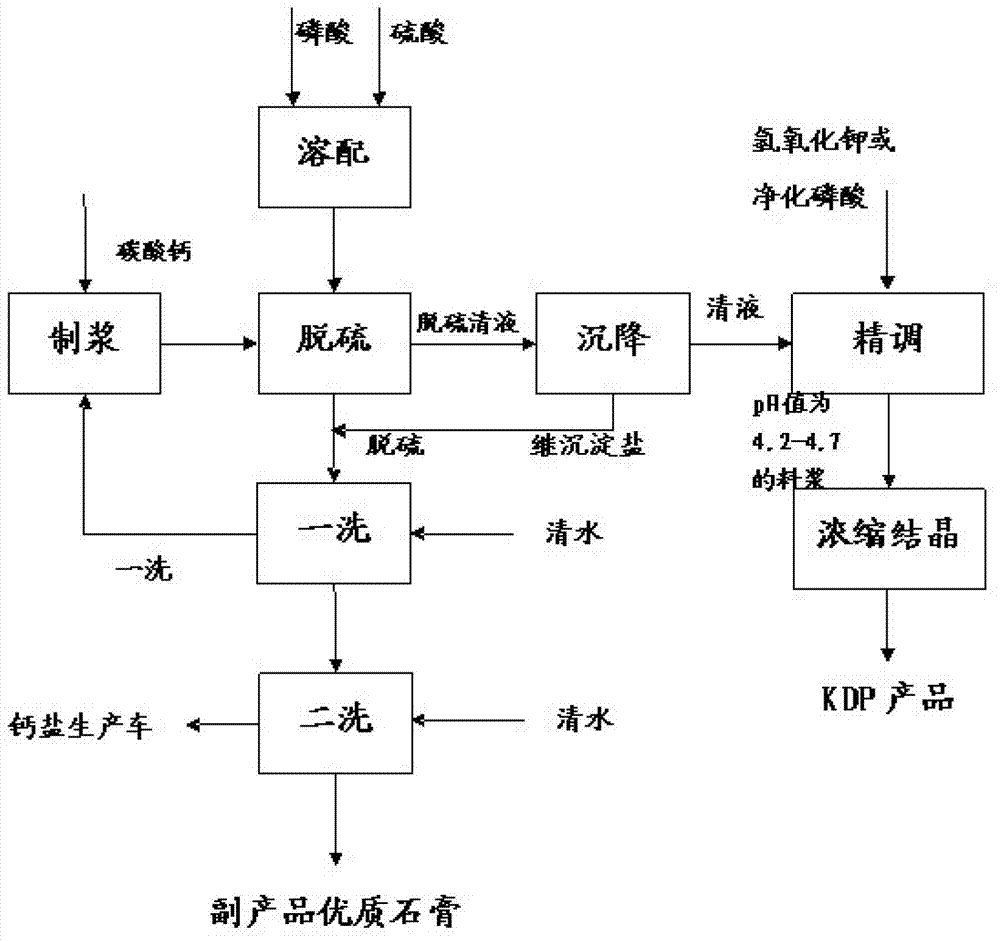
Image
Examples
Embodiment 1
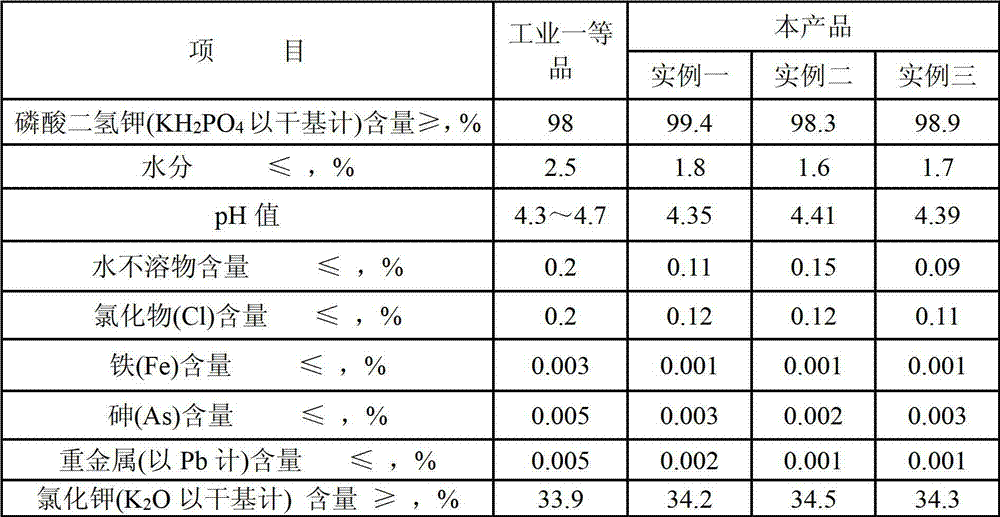
[0052] In this example P:K molar ratio=0.6:1, Ca:SO 4 Molar ratio=0.8︰1, the content of each component in the phosphoric acid purified by wet method is shown in Table 1:
[0053] Table 1 Various indicators of wet purification of phosphoric acid
[0054] project
P 2 o 5
Fe
As
Mg
F
SO 4
[0055] Index / %
20.12
0.0097
0.0014
0.03
0.0054
0.19
[0056] Potassium sulfate, potassium hydroxide, and calcium carbonate used in the experiment are all analytically pure, and the main content is above 98%;
[0057] (1) dissolving
[0058] Weigh 1000g of wet-purified phosphoric acid and add it to a reaction kettle with a heating device, start the agitator and heat up to 70-80°C, then weigh 420g of potassium sulfate and add it to dissolve, and keep it warm for 5-10 minutes after it is completely added to ensure complete dissolution , and each component exists in the form of ions;
[0059] (2) Pulping des...
Embodiment 2
[0073] In this example, P: K molar ratio = 1.1: 1, Ca: SO4 molar ratio = 0.9: 1, and the indicators of phosphoric acid purification by wet method are shown in Table 1.
[0074] (1) dissolving
[0075] Weigh 1000g of wet-process purified phosphoric acid and add it to a reaction kettle with a heating device, start the agitator and raise the temperature to 70-80°C, then weigh 229g of potassium sulfate and add it to dissolve, and keep it warm for 5-10 minutes after it is completely added to ensure complete dissolution , and each component exists in the form of ions;
[0076] (2) Pulping desulfurization
[0077] Take by weighing 118.31g of high-quality calcium carbonate powder, add 78.87g of water to make slurry, and configure calcium carbonate slurry with a solid content of about 60%;
[0078] Slowly add the slurry into the clear solution obtained in 1, and keep stirring the slurry to ensure that the air bubbles are completely removed.
[0079] Control the reaction temperature ...
Embodiment 3
[0090] In this example, P: K molar ratio = 1.2: 1, Ca: SO4 molar ratio = 1.1: 1, and the indicators of phosphoric acid purification by wet method are shown in Table 1.
[0091] (1) dissolving
[0092] Weigh 1000g of wet-purified phosphoric acid and add it to a reaction kettle with a heating device, start the agitator and raise the temperature to 70-80°C, then weigh 210g of potassium sulfate and add it to dissolve, and keep it warm for 5-10 minutes after it is completely added to ensure complete dissolution , and each component exists in the form of ions;
[0093] (2) Pulping desulfurization
[0094] Take by weighing 132.6g of high-quality calcium carbonate powder, add 88.3g of water to make slurry, and configure calcium carbonate slurry with a solid content of about 60%;
[0095] Slowly add the slurry into the clear solution obtained in 1, and keep stirring the slurry to ensure that the air bubbles are completely removed.
[0096] Control the reaction temperature of the sys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com