Calcitriol solid lipidic dispersion and preparation method thereof
A technology of calcitriol and solid lipid, which is applied in the field of medicine, can solve the problems of poor thermal stability of calcitriol, drug content of toxic and side effects, and cannot reach the therapeutic level, etc., and achieves high drug stability and high affinity. Sex, storage and consumption benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
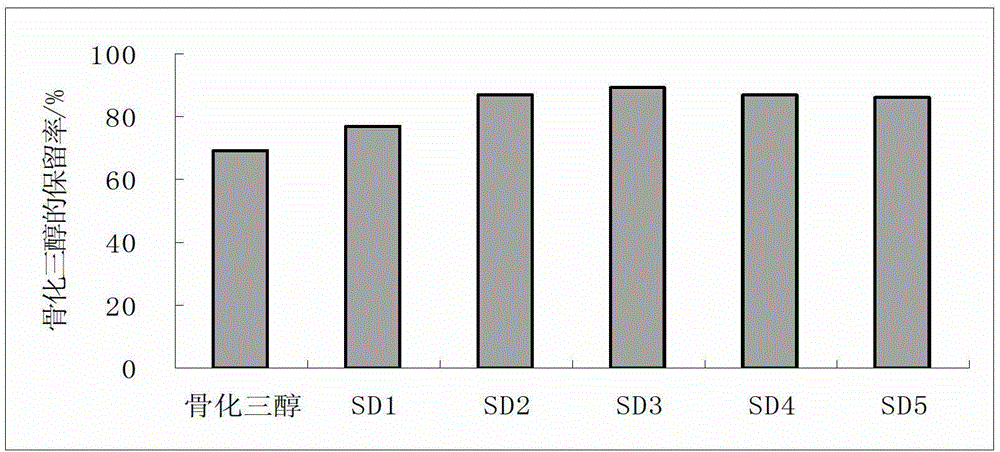
[0052] In this example, four calcitriol solid lipid dispersion samples were prepared, and the ratio of raw materials is shown in Table 2.
[0053] Preparation of calcitriol solid lipid dispersion: according to the ratio of raw materials listed in Table 2, add calcitriol and BHT to the molten lipid carrier, and dissolve calcitriol by vortexing and ultrasonication for 5-20 minutes Dispersed in the lipid carrier to obtain the calcitriol lipid mixture; quickly add the calcitriol lipid mixture in the molten state to the solid carrier for adsorption and solidification.
[0054] Stability test: samples SLD1, SLD2, SLD3, and SLD4 were placed in a light-proof oven at 40°C, and samples were taken on the 10th day, and the retention rate of calcitriol in the samples was measured by high performance liquid chromatography.
[0055] Table 2 Raw material ratio (mass ratio) of calcitriol solid lipid dispersion
[0056]
[0057]
[0058] The stability test result comparison of calcitriol...
Embodiment 2
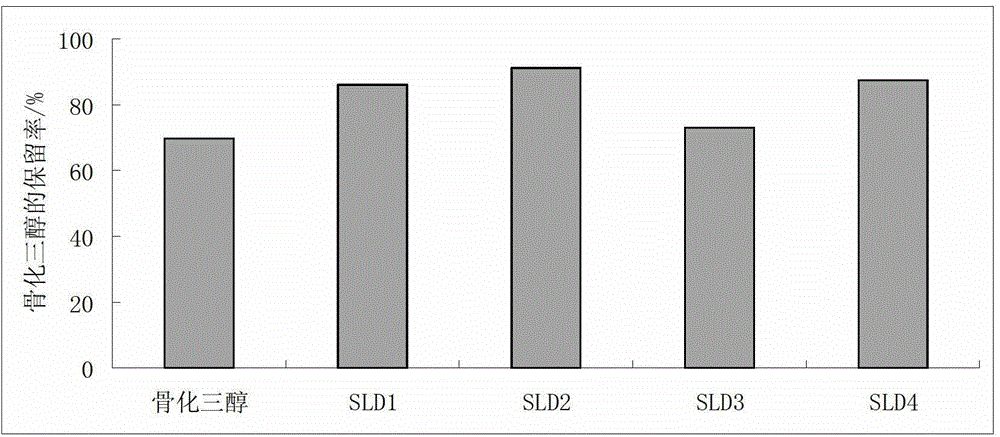
[0060] In this example, 5 calcitriol solid lipid dispersions were prepared, and their raw material ratios are shown in Table 3, and the preparation method was the same as in Example 1. The difference is that the 5 calcitriol solid lipid dispersions in this example use 2 kinds of lipid carriers. The stability test method is the same as in Example 1.
[0061] Table 3 Raw material ratio (mass ratio) of calcitriol solid lipid dispersion
[0062]
[0063] The stability test results of calcitriol in 5 calcitriol solid lipid dispersions prepared by the present embodiment are compared as figure 2 , Table 5. From figure 2 , Table 5 As can be seen, the stability of SLD5-SLD9 is improved by nearly 20% compared with the bulk drug; wherein SLD8-SLD9 is equivalent to SLD2 in Example 1, and after SLD5-SLD7 is added with the lipid carrier TPGS, the stability is higher than that of the implementation SLD2 in Example 1 is better.
Embodiment 3
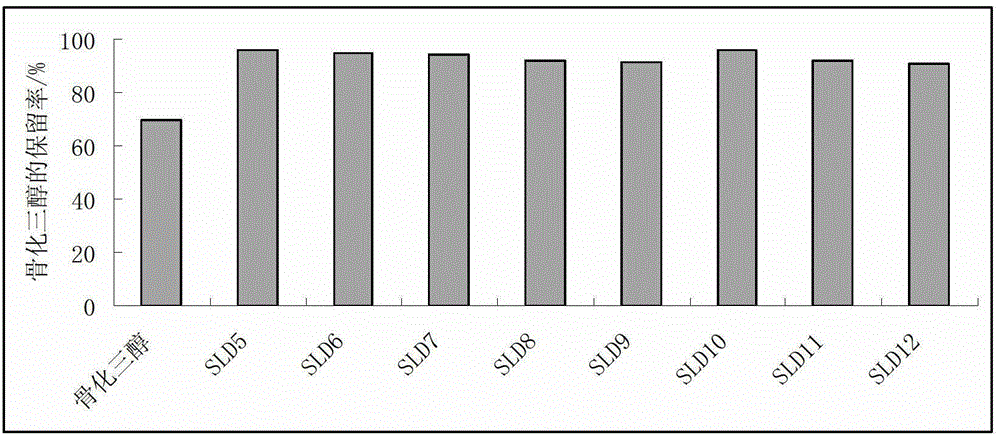
[0065] In this example, three calcitriol solid lipid dispersions were prepared, and the ratio of raw materials was shown in Table 4, and the preparation method was the same as in Example 1. The difference is that the three calcitriol solid lipid dispersions in this example use three kinds of lipid carriers. The stability test method is the same as in Example 1.
[0066] Table 4 Raw material ratio (mass ratio) of calcitriol solid lipid dispersion
[0067]
[0068] The stability test results of calcitriol in 3 calcitriol solid lipid dispersions prepared by the present embodiment are compared as follows image 3 , Table 5. As can be seen from Table 5, the stability of SLD10-SLD12 is improved by nearly 20% compared with the bulk drug; wherein SLD12 is equivalent to SLD2 in Example 1, and after SLD10 and SLD11 are added with lipid carrier TPGS, the stability is better than that in Example 1 SLD2 is better.
[0069] Table 5 Stability of Calcitriol Solid Dispersion at 40°C Air...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com