Self-assembly medicine vesicles and construction method thereof
A construction method and self-assembly technology, which is applied in the fields of biomedicine and physical chemistry, can solve problems such as difficult formation of precipitates, and achieve the effects of broadening the scope of research, simple methods, and easy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of cytarabine-1,4-bis(2-ethylhexyl)succinate sodium sulfonate (AOT) ion-pair amphiphile self-assembled vesicles
[0047] (1) Accurately weigh 0.5594 g of cytarabine hydrochloride with an analytical balance, add 2 mL of water, the molar concentration is 1 mol / L, and dissolve by ultrasonication for 10 s;
[0048] (2) Accurately weigh 0.8891 g of the surfactant 1,4-bis(2-ethylhexyl) succinate sodium sulfonate (AOT) with an analytical balance and measure 18 mL of water to prepare a molar concentration of 0.1 mol / L, placed in a clean beaker, ultrasonicated for 10 minutes, stirred at 72°C under the action of a magnetic stirrer at a stirring speed of 300 rpm, and stirred for 0.5h until AOT was completely dissolved to obtain an AOT solution;
[0049] (3) Add the AOT solution dropwise to the cytarabine solution at 72°C under the action of a magnetic stirrer at a stirring speed of 200 r / min. The molar ratio of AOT to cytarabine hydrochloride is 1:1, stir for 1 hour ...
Embodiment 2
[0056] Drug-Surfactant Ionic Structure Confirmation
[0057] The Cl in the cytarabine-AOT ion pair (Cytarabine-AOT) prepared in Example 1 was analyzed by silver nitrate precipitation method. - Qualitative detection showed that no precipitate was formed, indicating that the Cytarabine-AOT ion pair (Cytarabine-AOT) no longer contained NaCl.
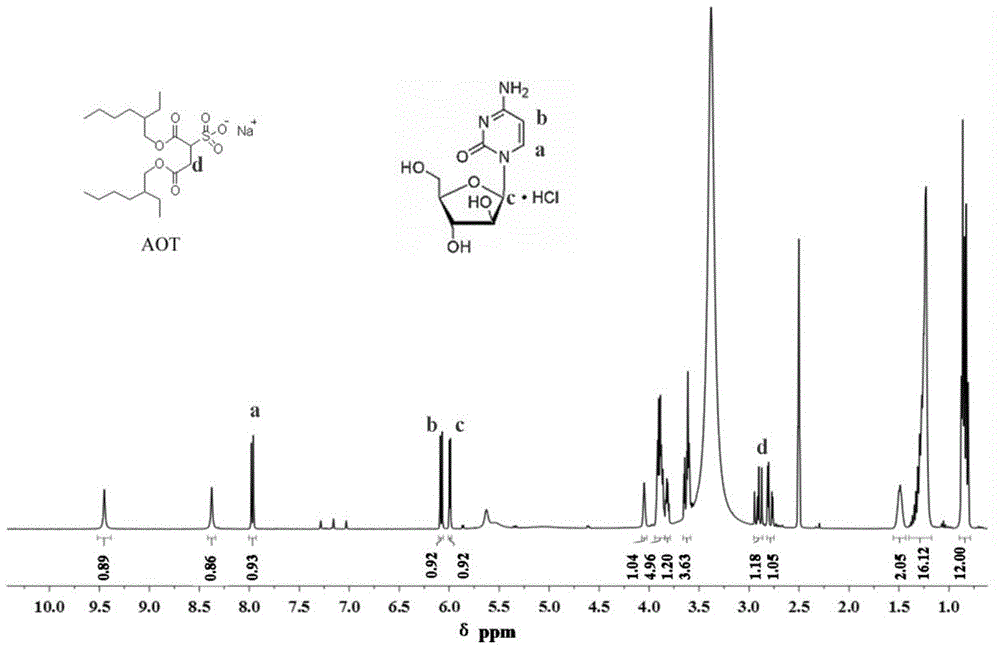
[0058] NMR 1 The chemical composition of cytarabine-AOT ion pair (Cytarabine-AOT) was confirmed by the method of H spectrum, and the results are as follows figure 1 As shown, the molar ratio of drug to surfactant in ion pairing is 1:1.
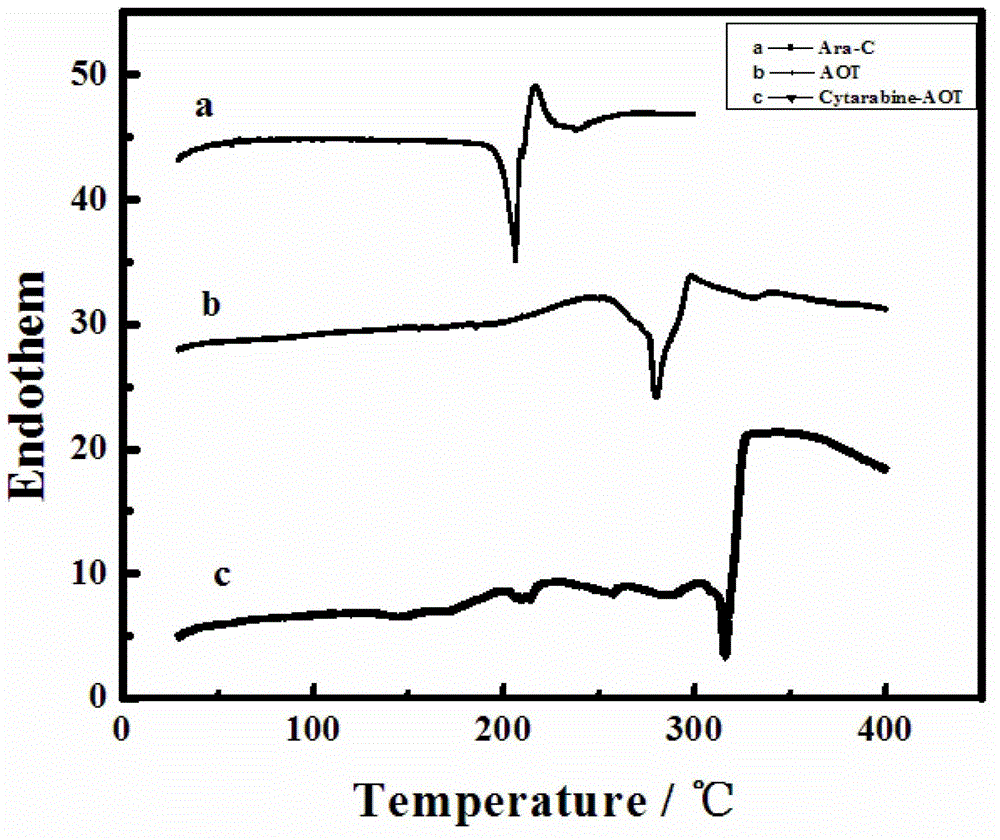
[0059] The phase transfer behavior of cytarabine hydrochloride, AOT, cytarabine hydrochloride-AOT ion pair (Cytarabine-AOT) is measured respectively by DSC analysis method, the results are as follows figure 2 As shown, the appearance of new endothermic peaks proves the formation of new substances.
Embodiment 3
[0061] Study on Aggregation Behavior of Cytarabine-AOT
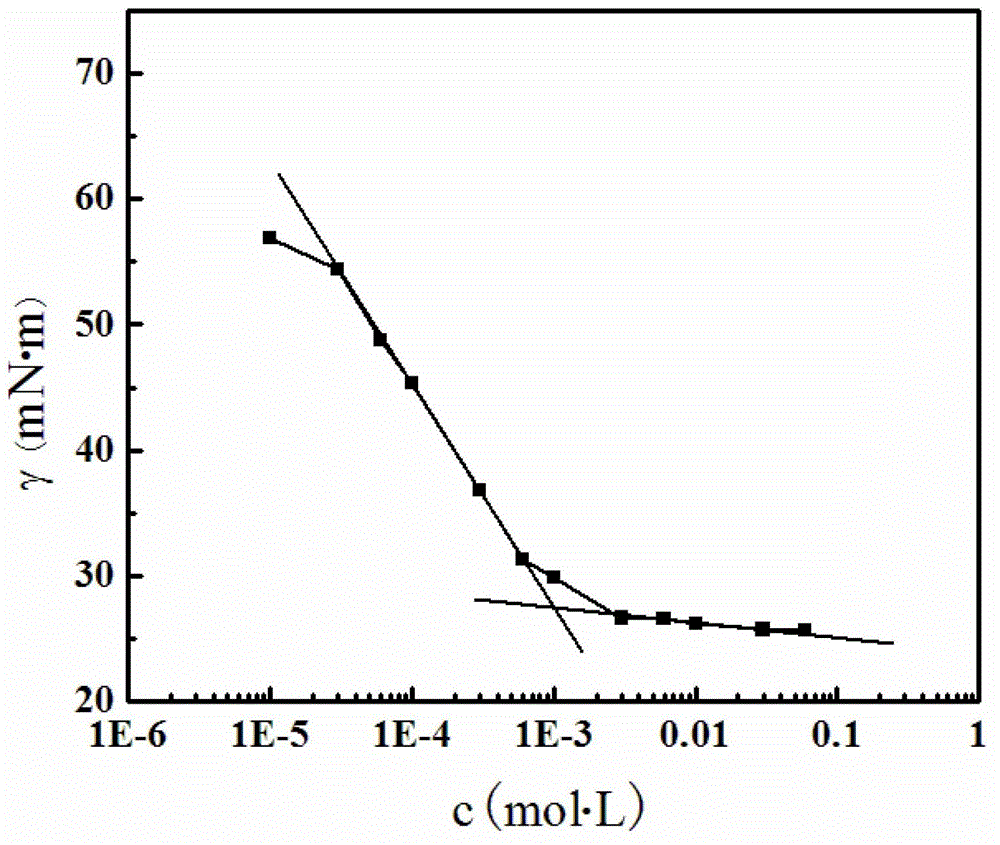
[0062] The molar concentration of the preparation is 10 -5 ~10 -2 A series of ion-pair solutions of mol / L, using a surface tensiometer, 25 ℃, the surface tension of the sample is measured by the hanging method, the results are as follows image 3 As shown, ions have high surface activity to amphiphilic molecules, which can make the surface tension of water from 70mN·m -1 reduced to 26mN·m -1 , with a critical aggregation concentration of about 1×10 -3 mol / L.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com