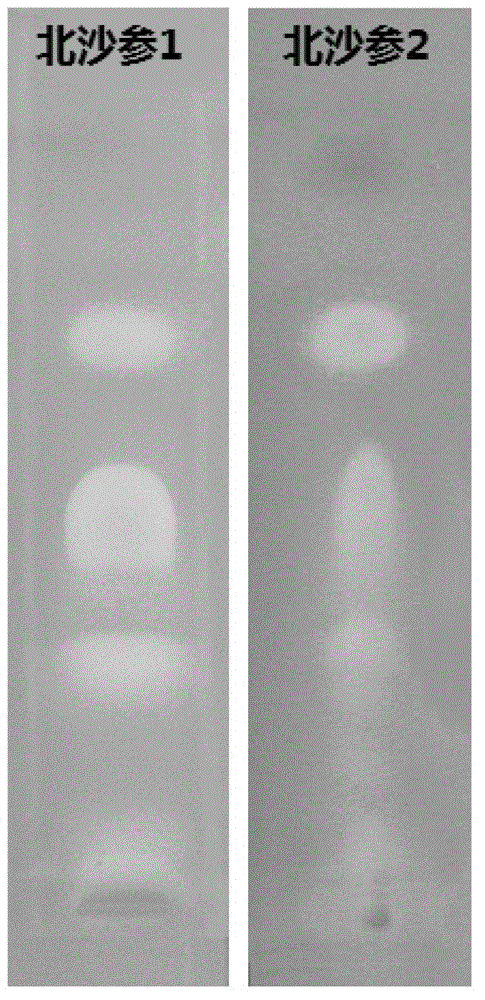
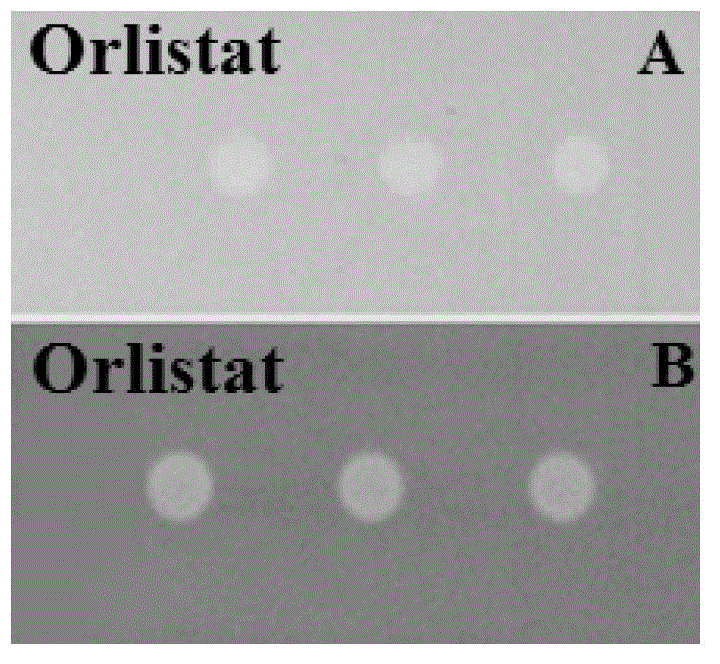
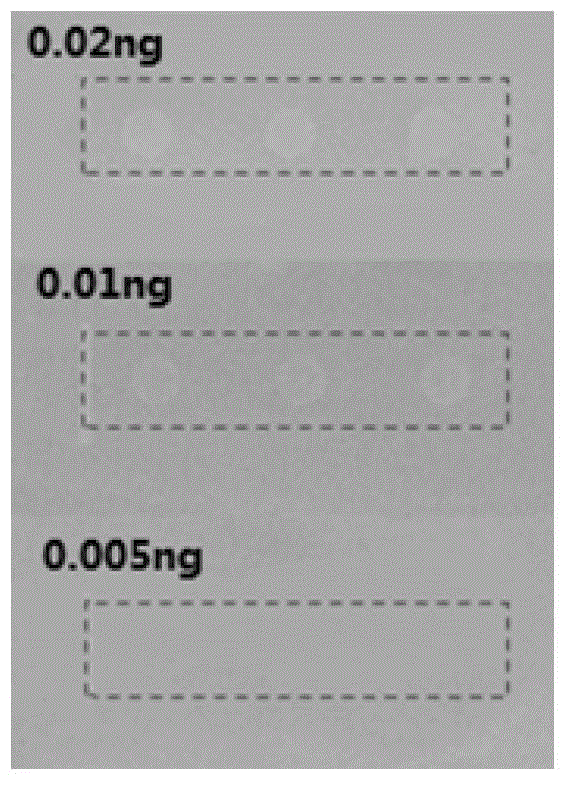
Method for screening lipase inhibitor
A technology of lipase inhibitors and screening methods, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that cannot be adapted to the simultaneous screening of drugs, the range of test samples is small, and the effective ingredients are not clear, so as to achieve easy identification and memory, low experimental cost and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of enzymatic buffer solution: Accurately weigh 1.212g of tris hydroxymethylaminomethane powder and 0.455g of anhydrous calcium chloride powder as a solvent in deionized water, adjust the pH value of the mixture to 6.5 with hydrochloric acid, and dissolve The solution was finally adjusted to 500mL, prepared into an enzymatic reaction buffer (pH=6.5) with a tris concentration of 20mmol / L, and stored in a refrigerator at 2-4°C for future use.
[0029] Preparation of substrate solution: 2-naphthyl myristate powder was dissolved in petroleum ether to prepare a substrate solution with a concentration of 16 mmol / L.
[0030] Preparation of lipase solution: Add porcine pancreatic lipase to the above enzymatic buffer to prepare a solution with a concentration of 10u / mL.
[0031] Preparation of developer solution: Dissolve Fast Blue B salt powder in deionized water to prepare a developer solution with a concentration of 0.5 mg / mL.
[0032] Preparation of the test solu...
Embodiment 2
[0037] Preparation of enzymatic buffer solution: Accurately weigh 3.029g of Tris powder and 0.91g of anhydrous calcium chloride powder as a solvent in deionized water, adjust the pH value of the mixed solution to 7.8 with hydrochloric acid, and dissolve The solution was finally adjusted to 500mL, prepared into 50mmol / L enzymatic reaction buffer (PH=7.8), and stored in a refrigerator at 2-4°C.
[0038] Preparation of substrate solution: 2-naphthyl myristate powder was dissolved in petroleum ether to prepare substrate solutions with concentrations of 0.25mmol / L and 350mmol / L respectively.
[0039] Lipase solution: Add porcine pancreatic lipase to the above-mentioned enzymatic buffer solution to prepare solutions with concentrations of 5u / ml and 200u / ml respectively.
[0040] Preparation of developer solution: Dissolve Fast Blue B salt powder in deionized water to prepare developer solutions with concentrations of 0.1 mg / ml and 100 mg / ml respectively.
[0041]Preparation of the ...
Embodiment 3
[0047] Preparation of enzymatic buffer solution: Accurately weigh 3.029g of Tris powder and 0.91g of anhydrous calcium chloride powder in deionized water, adjust the pH value of the mixture to 7.0 with hydrochloric acid, and dissolve The solution was finally adjusted to 500mL, prepared into 10mmol / L enzymatic reaction buffer (PH=7.0), and stored in a refrigerator at 2-4°C.
[0048] Preparation of substrate solution: 2-naphthyl myristate powder was dissolved in petroleum ether to prepare a substrate solution with a concentration of 25 mmol / L.
[0049] Lipase solution: add porcine pancreatic lipase to the above enzymatic buffer to prepare a solution with a concentration of 35u / ml.
[0050] Preparation of developer solution: Dissolve Fast Blue B salt powder in deionized water to prepare a developer solution with a concentration of 0.2 mg / ml.
[0051] Preparation of the test solution: Dissolve Orlistat (Orlistat, O4139-25MG) powder in 95% ethanol to prepare 4×10 -8 mol / L, 2×10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com