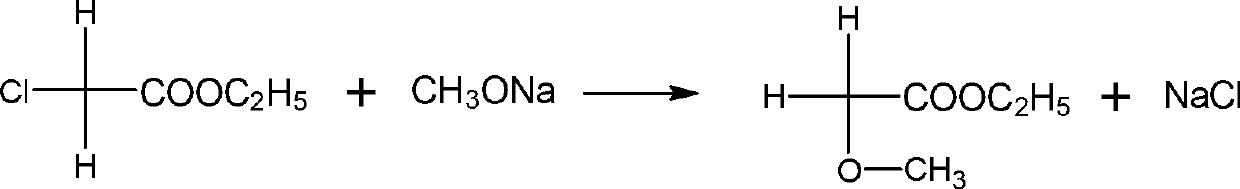
Synthesis method of 2,4-dichlorophenyl acetaldehyde
A technology of dichlorophenylacetaldehyde and synthesis method, which is applied in the preparation of heterocyclic compounds, organic chemistry, etc., can solve the problems of reducing the content of 2,4-dichlorophenylacetaldehyde, increase the content and yield, and avoid side effects. Response, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
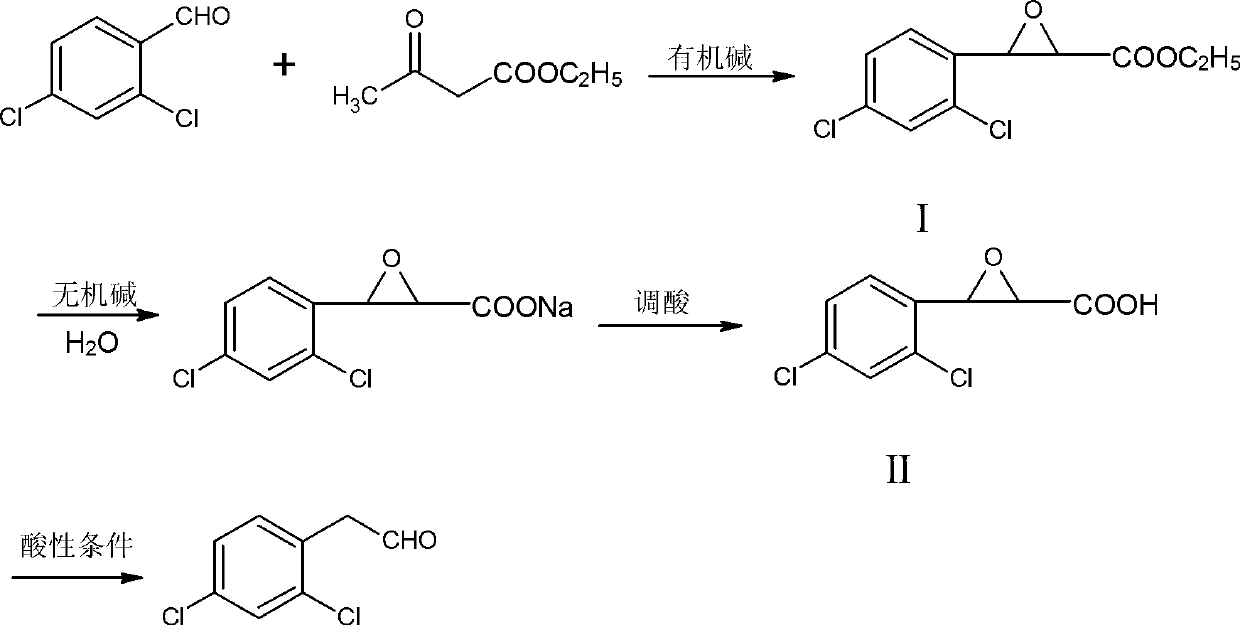
[0025] A kind of synthetic method of 2,4-dichlorophenylacetaldehyde, its concrete steps are:
[0026] (1) Closed loop
[0027] Add 118.9g of sodium methoxide solution (5% mass fraction) to 50ml of methanol, stir evenly, add 17.5g of 2,4-dichlorobenzaldehyde, stir until completely dissolved, then add 14.3g of ethyl acetoacetate dropwise, drop After completion, the reaction was carried out at 60° C., followed by TLC until the reaction was complete. After filtration, the filtrate was cooled to -5° C. for crystallization, and 22.7 g of solid intermediate I was obtained by suction filtration, with a content of 85% and a yield of 87%.
[0028] (2) Alkaline hydrolysis
[0029] Add 34.6g potassium carbonate aqueous solution (mass fraction: 20%) to 13.1g intermediate product I, keep the temperature at 20°C for reaction, TLC tracking detection until the reaction is complete;
[0030] (3) Acid adjustment
[0031] The above-mentioned completely reacted solution was lowered to room temp...
Embodiment 2
[0035] A kind of synthetic method of 2,4-dichlorophenylacetaldehyde, its concrete steps are:
[0036] (1) Closed loop
[0037] Add 907g of sodium ethoxide solution (mass fraction: 15%) to 200ml of ethanol, stir evenly, add 175g of 2,4-dichlorobenzaldehyde, stir until completely dissolved, then add 390g of ethyl acetoacetate dropwise, dropwise, 40 ℃ heat preservation reaction, TLC tracking detection until the reaction is complete, filter, the filtrate is cooled to -5 ℃ for crystallization, suction filtration to obtain 229.8 g of solid intermediate product I, the content is 83%, and the yield is 88%.
[0038] (2) Alkaline hydrolysis
[0039] Add 212.7g of sodium carbonate aqueous solution (5% mass fraction) to 13.1g of intermediate product I, keep the reaction at 40°C, and track and detect by TLC until the reaction is complete;
[0040] (3) Acid adjustment
[0041] The above-mentioned fully reacted solution was lowered to room temperature, acidified with acetic acid until the...
Embodiment 3
[0045] A kind of synthetic method of 2,4-dichlorophenylacetaldehyde, its concrete steps are:
[0046] (1) Closed loop
[0047] Add 123.1g of sodium isopropoxide solution (mass fraction: 10%) to 80ml of isopropanol, stir evenly, add 17.5g of 2,4-dichlorobenzaldehyde, stir until completely dissolved, then add dropwise 26g of ethyl acetoacetate Ester, dripping, 50 ℃ heat preservation reaction, TLC tracking detection until the reaction is complete, filtration, the filtrate cooled to -5 ℃ crystallization, suction filtration to obtain 22.5g of solid intermediate product I, the content is 81%, the yield is 86%.
[0048] (2) Alkaline hydrolysis
[0049] Add 63.2g sodium bicarbonate aqueous solution (mass fraction is 10%) to 13.1g intermediate product I, 30 DEG C of heat preservation reaction, TLC track detection until reaction is complete;
[0050] (3) Acid adjustment
[0051] The above-mentioned fully reacted solution was lowered to room temperature, acidified with hydrochloric ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com