Antihypertensive Tablets Containing Sodium Chloride Drug Carrier
A sodium chloride and blood pressure-lowering technology, applied in the field of medicine, can solve the problems of degradation, inappropriate use of excipients, affecting the stability of candesartan medoxomil, etc., and achieve the effects of fast release, inhibition of crystal form confusion, and excellent compression moldability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Preparation of candesartan cilexetil tablets
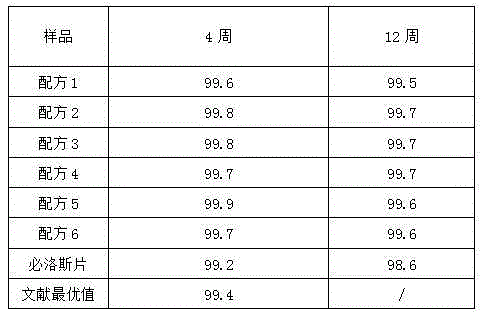
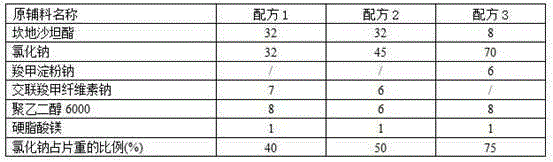
[0016] 1. Recipe: See Table 1
[0017] Table 1 formula of candesartan cilexetil tablets (1000 tablets, unit: g)
[0018]
[0019] 2. Preparation:
[0020] 1) Weigh polyethylene glycol 6000 according to the proportion of the formula, heat up to 70~75°C, and melt into a liquid ( ).
[0021] 2) Weigh candesartan cilexetil, sodium chloride, and croscarmellose sodium (or carboxymethyl starch sodium) according to the ratio of the formula, mix them evenly, add them into the turbine granulator, turn on the turbine granulator, and control The inlet air temperature keeps the material temperature at 25~40°C, and the ( ) is slowly sprayed onto the material by air-flow atomization, discharges the material after spraying, and granulates to obtain candesartan cilexetil granules ( ).
[0022] 3) take magnesium stearate and ( ) mixed evenly, at 15kN / cm 2 The following pressure tablet.
Embodiment 2
[0023] Example 2 Preparation of candesartan cilexetil tablets
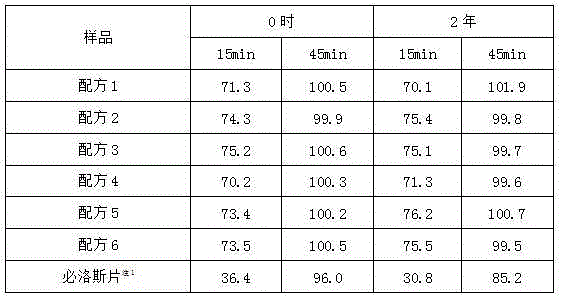
[0024] 1. Formulation: See Table 2
[0025] Table 2 formula of candesartan cilexetil tablets (1000 tablets, unit: g)
[0026]
[0027] 2. Preparation Process:
[0028] 1) Weigh polyethylene glycol 8000 according to the proportion of the formula, heat up to 70~80°C, and melt into a liquid ( ).
[0029] 2) Weigh candesartan cilexetil, sodium chloride, and croscarmellose sodium (or carboxymethyl starch sodium) according to the ratio of the formula, mix them evenly, add them into the turbine granulator, turn on the turbine granulator, and control The inlet air temperature keeps the material temperature at 25~40°C, and the ( ) is slowly sprayed onto the material by air-flow atomization, discharges the material after spraying, and granulates to obtain candesartan cilexetil granules ( ).
[0030] 3) take magnesium stearate and ( ) mixed evenly, at 15kN / cm 2 The following pressure tablet.
[0031]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com