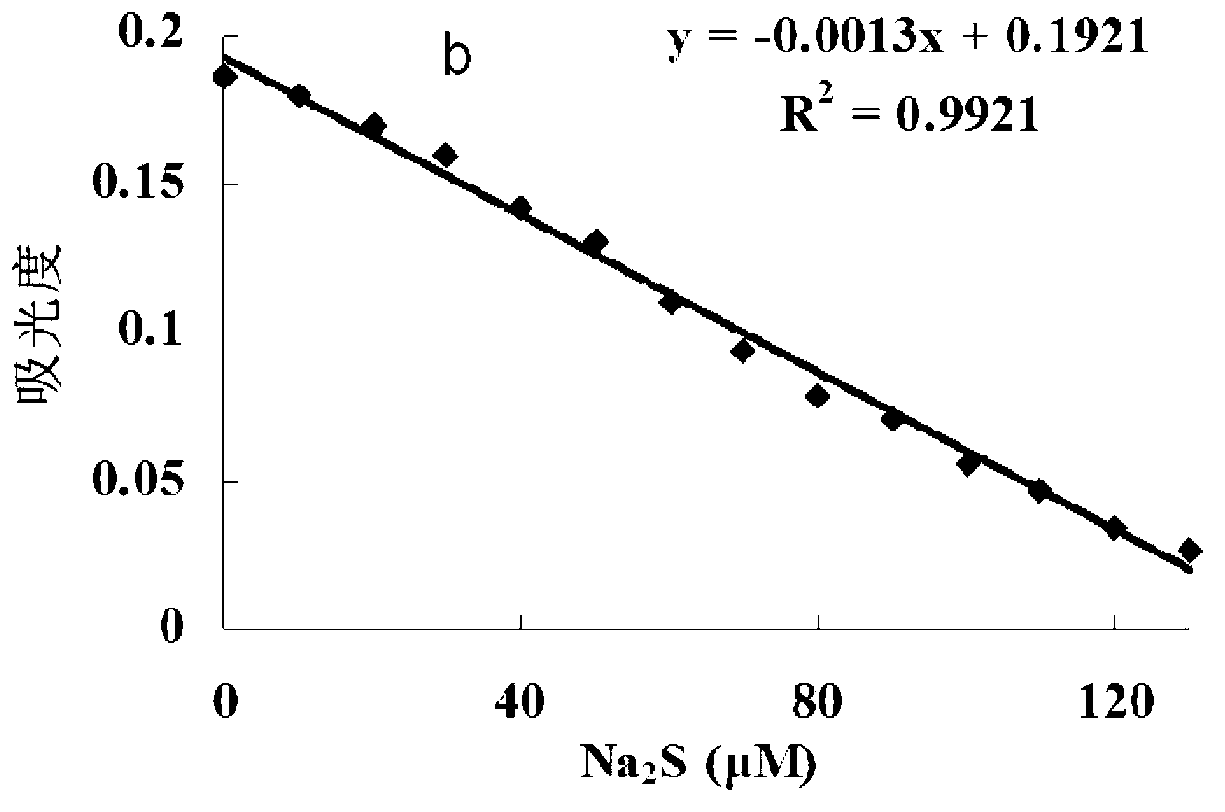Method for preparing hydrogen sulphide fast and highly selectively colorimetric probe
A technology of colorimetric probe and hydrogen sulfide, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of carboxylic acid nitriles. Simple, water-soluble effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] (Scheme 1) Dissolve 377.3mg (1mmol) of nitrogen-phenyldiethanolamine and 128mg (1.0mmol) of tetracyanoethylene in 20mL of N,N-dimethylformamide (DMF), then heat and reflux at 80°C for 4h, Then use ethyl acetate to extract to get the ethyl acetate phase, and then rotary evaporate to get the crude product, then use the mixed system of dichloromethane and anhydrous methanol (v / v, 100:1) for column chromatography to get the red pure product 298mg, the yield was 59%.
[0037] (Scheme 2) Dissolve 377.3mg (1mmol) of nitrogen-phenyldiethanolamine and 192mg (1.5mmol) of tetracyanoethylene in 20mL of N,N-dimethylformamide (DMF), then heat and reflux at 80°C for 4h, Then use ethyl acetate to extract to get the ethyl acetate phase, and then rotary evaporate to get the crude product, then use the mixed system of dichloromethane and anhydrous methanol (v / v, 100:1) for column chromatography to get the red pure product 415mg, the yield was 82%.
[0038](Scheme 3) Dissolve...
Embodiment 2
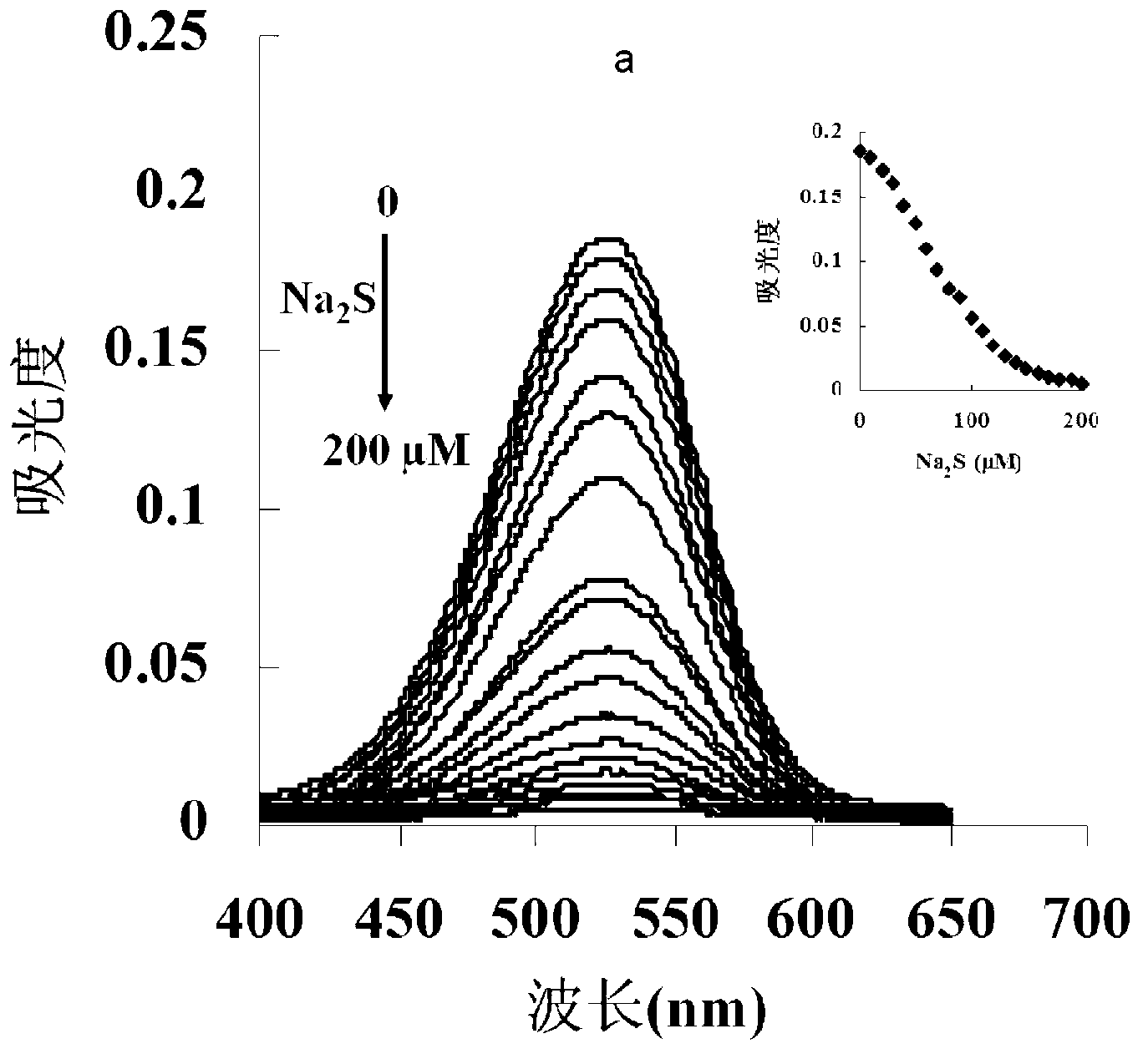
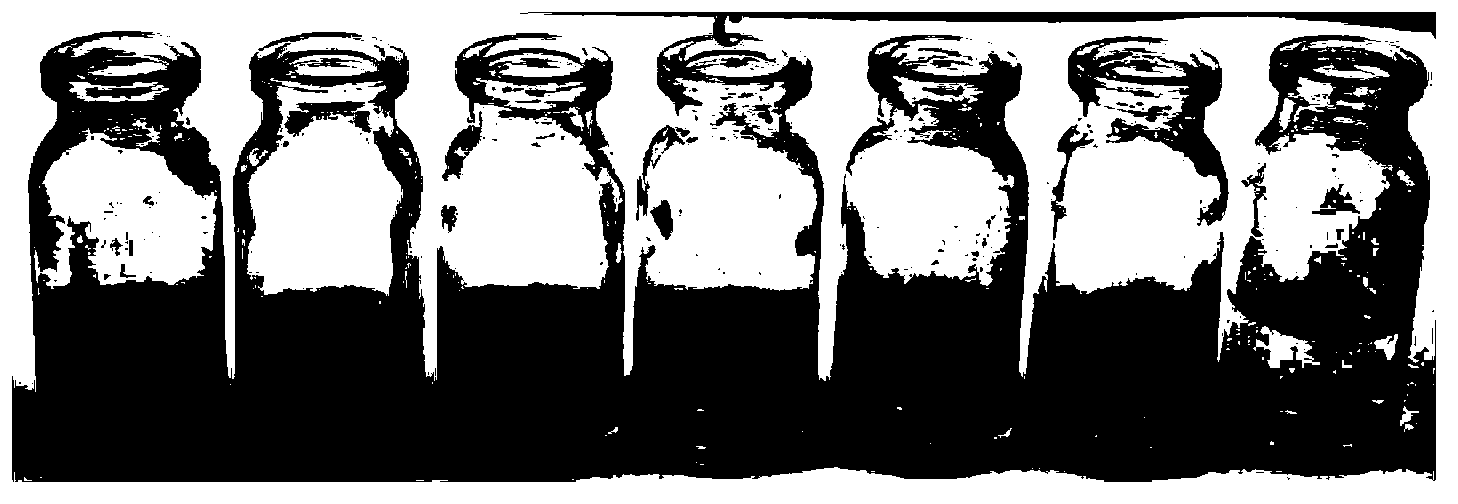
[0043] The inventor of the present invention has carried out following test: (a) different concentration Na 2 Effect of S (0-200μM) on the absorption spectrum of the probe (5μM); (b) the relationship between the absorption intensity at 527nm and the added Na 2 The linear relationship between S concentration (0-130μM); (c) different concentrations of Na 2 The color of the probe solution (5 μM) changed from pink to colorless in the presence of S (from left to right: 0, 50, 100, 200, 300, 400, 500 μM). The above determination was carried out in water (20mM PBS, pH 7.4), the probe used was the probe prepared in Example 1, and all spectral tests were carried out at 25°C under Na 2 Measured after adding S for 10 min. See Figure 1 for the results.
[0044] It can be seen from Figure 1 that with the Na in the probe solution 2 With the increase of S concentration, the absorption spectrum decreased gradually, and at 0 - 130 μM Na 2 In the concentration range of S, Na 2 The concen...
Embodiment 3
[0046] The effect of different analytes (150 μM) on the absorption spectrum of the probe (5 μM). Analytes include: cysteine Cys, glutathione GSH, leucine Leu, proline Pro, threonine Thr, glutamic acid Glu, glycine Gly, potassium ion K + , calcium ion Ca 2+ , Zinc ion Zn 2+ , Sulfate ion SO 4 2- , nitrate ion NO 3 - , perchlorate ion ClO 4 - , Sodium Sulfide Na 2 S, their concentration is 150 μM. All test conditions were completed in water (20mM PBS, pH 7.4), the probe used was the probe prepared in Example 1, and all spectra were measured after adding the analyte at 25°C for 10 minutes . Specifically, pipette 25 μL of the probe stock solution (1 mM) into a 5 mL colorimetric tube, then add 3 mL of ultrapure water, then pipette 75 μL of the above analyte stock solution (10 mM) into the colorimetric tube, and then pipette 0.5 mL of PBS solution (pH 7.4, 200mM), and finally dilute to 5mL with ultrapure water. Shake well, let it stand for 10min, then measure. The res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com