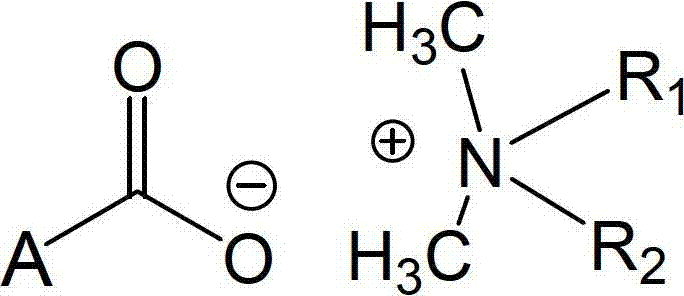
Amino-acid dual-chain quaternary-amino carboxylate, preparation method and application in microbicides thereof
A technology of quaternary amino carboxylate and didecyl dimethyl amino carboxylate, which is applied in the field of broad-spectrum, high-efficiency microorganism-killing new compounds and their preparation, and can solve problems such as mutagenesis and reproductive defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Production of Pyroglutamic Acid Didecyl Dimethylaminocarboxylate DAPC (N, N-di-n-decyl-N, N-dimethyl-ammonium 5-oxopyrrolidine-2-carboxylate)
[0075] 1) First weigh 0.03 mol of sodium hydroxide, dissolve it in deionized water to make the solution weigh 30 g, and cool it down.
[0076] 2) Weigh again 0.03 mol of pyroglutamic acid and dissolve it in the above-mentioned 30 g of sodium hydroxide aqueous solution. The reaction product is an aqueous solution of sodium pyroglutamate (solution A).
[0077] 3) Weigh 0.025 mol of didecyl dimethyl ammonium chloride and dissolve it in 160 g of deionized water to obtain solution B.
[0078] 4) Mix liquid A and liquid B thoroughly to obtain solution C.
[0079] 5) The mass of solution C is about 200g.
[0080] 6) Take 200g of solution C, add 400ml of dichloromethane, and add 10g of sodium chloride, stir well, put it into an extraction bottle, shake well, and let it stand for 30 minutes.
[0081] 7) Remove the lower layer and vac...
Embodiment 2
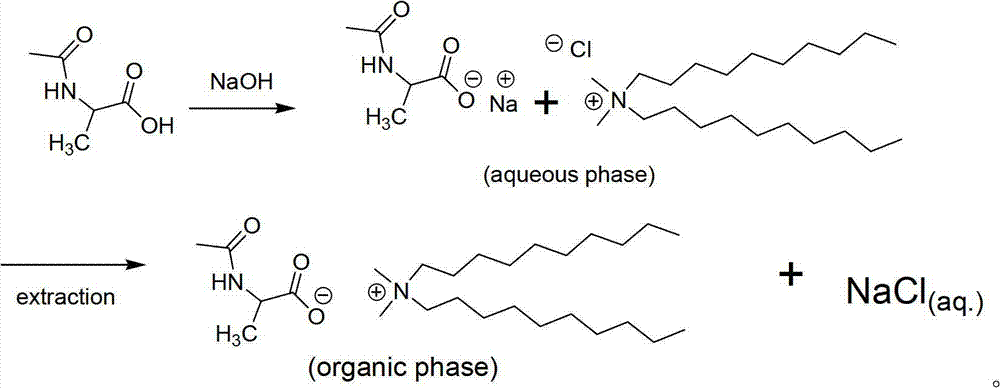
[0085] Make alanine didecyl dimethyl amino carboxylate DAAC (N, N-din-decyl-N, N-dimethyl-ammonium N-acetylated alanine carboxylate)
[0086] (1) First weigh 0.03 mol of sodium hydroxide, dissolve it in deionized water to make the solution weigh 30 g, and cool it.
[0087] (2) Weigh again 0.03 mol of acetylated alanine and dissolve it in 30 g of sodium hydroxide aqueous solution. The reaction product is an aqueous solution of sodium pyroglutamate (solution A).
[0088] (3) Weigh 0.025 mol of didecyl dimethyl ammonium chloride and dissolve it in 160 g of deionized water to obtain solution B.
[0089] (4) Thoroughly mix liquid A and liquid B to obtain solution C.
[0090] (5) The mass of solution C is about 200g.
[0091] (6) Take 200 g of solution C, add 400 ml of dichloromethane, and add 10 g of sodium chloride, stir well, put it into an extraction bottle, shake well, and let it stand for 30 minutes.
[0092] (7) Remove the lower layer liquid and vacuum rotary evaporate fo...
Embodiment 3
[0096] Other amino acid double-chain quaternary amino carboxylates can be prepared by the same method, such as:
[0097] N, N-dimethyl-N, N-di-n-decylammonium N-acetylated proline carboxylate
[0098]
[0099] N, N-dimethyl-N, N-di-n-decylammonium N-acetylated phenylalanine carboxylate
[0100]
[0101] N, N-dimethyl-N, N-di-n-decylammonium N-acetylated serine carboxylate
[0102]
[0103] N, N-dimethyl-N, N-di-n-decylammonium N-acetylated isoleucine carboxylate
[0104]
[0105] N, N-dimethyl-N, N-di-n-decylammonium N-acetylated S-methyl cystine carboxylate
[0106]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com