Water-soluble taxol polymer with tumor actively-targeted property
A technology of compounds and taxanes, applied in the field of medicine, can solve the problems of undisclosed preparation methods and characterization data, and achieve the effect of improving active selectivity, high curative effect, and high curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 prepares polyglutamic acid
[0058] Dissolve 54.0g of triphosgene in 300mL of anhydrous tetrahydrofuran, add 50.0g of L-glutamic acid-γ benzyl ester in three batches at room temperature, exothermic heat rises in the system, add ice water bath to cool if necessary, after the reaction is stable, oil The temperature of the bath was raised to 50°C, and after all the solids were dissolved, the temperature was kept for half an hour. The solvent was removed by rotary evaporation under reduced pressure to obtain an off-white or light yellow solid, which was recrystallized by adding a mixed solvent of 150mL tetrahydrofuran and 200mL petroleum ether hot at 50°C, recrystallized again according to the above solvent ratio, filtered by suction, and dried under reduced pressure at room temperature for 12 Hours, 22.7g white solid was obtained, yield: 40.9%, mp: 91.4-92.7°C. 1 HNMR (500MHz, CDCl 3 ): δ7.25~7.38(m, 5H, Ar-H), 6.71(s, 1H, -NH), 5.13(s, 2H, -CH 2 ), 4.37(t,...
Embodiment 2
[0061] Embodiment 2: Preparation of polyglutamic acid paclitaxel sodium salt
[0062] Dissolve 0.3g polyglutamic acid in 7.5mL N,N-dimethylformamide, add 0.1g paclitaxel, 0.1g EDC and a small amount of DMAP, keep away from light, stir at room temperature for 6 hours, detect by thin layer chromatography, develop The agent was dichloromethane:methanol=95:5 (v / v), and paclitaxel disappeared completely. The reaction solution was poured into 500 mL of dichloromethane for eluting, suction filtered, rinsed, and vacuum-dried in the dark at room temperature for 6 hours. 0.33 g of off-white poly-paclitaxel was obtained. Yield 82.5%. By changing the weight ratio of paclitaxel and polyglutamic acid in the starting material, polyglutamic acid paclitaxel containing different concentrations of paclitaxel can be synthesized.
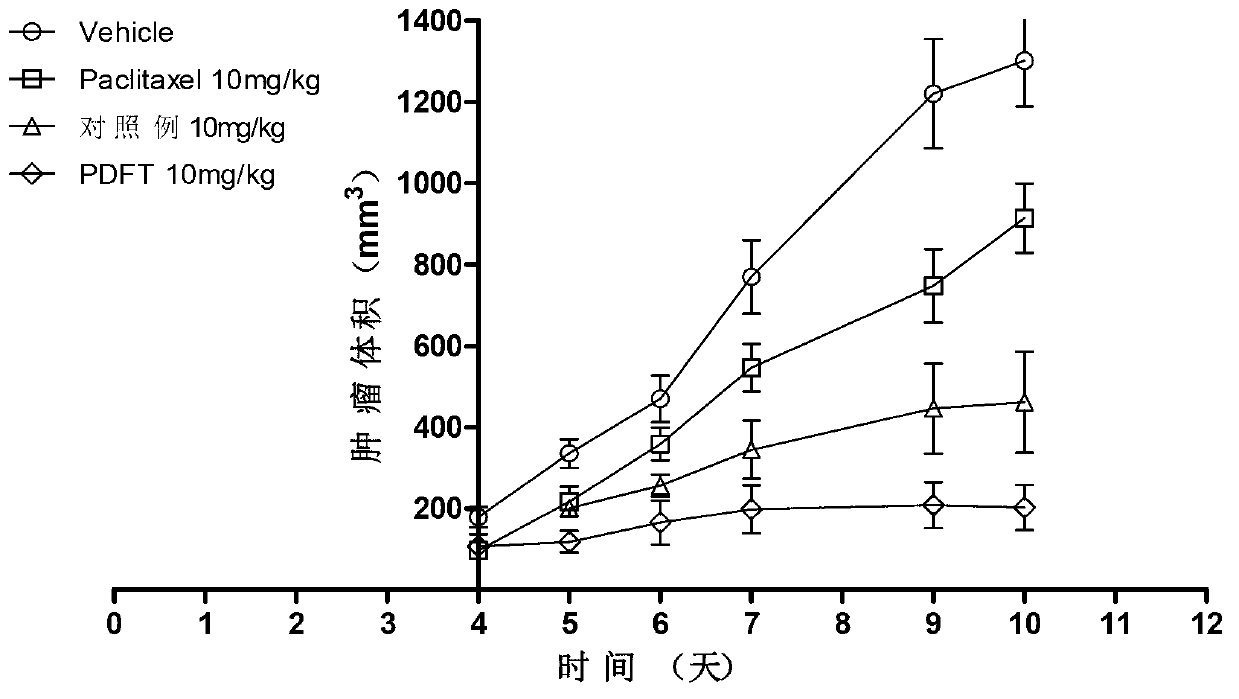
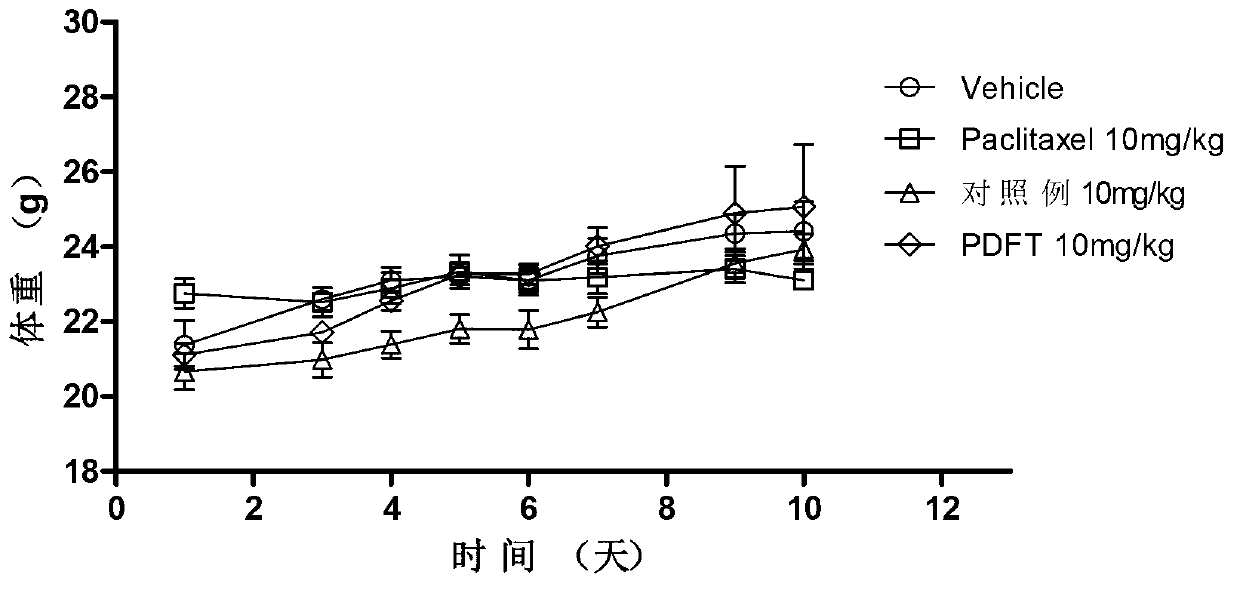
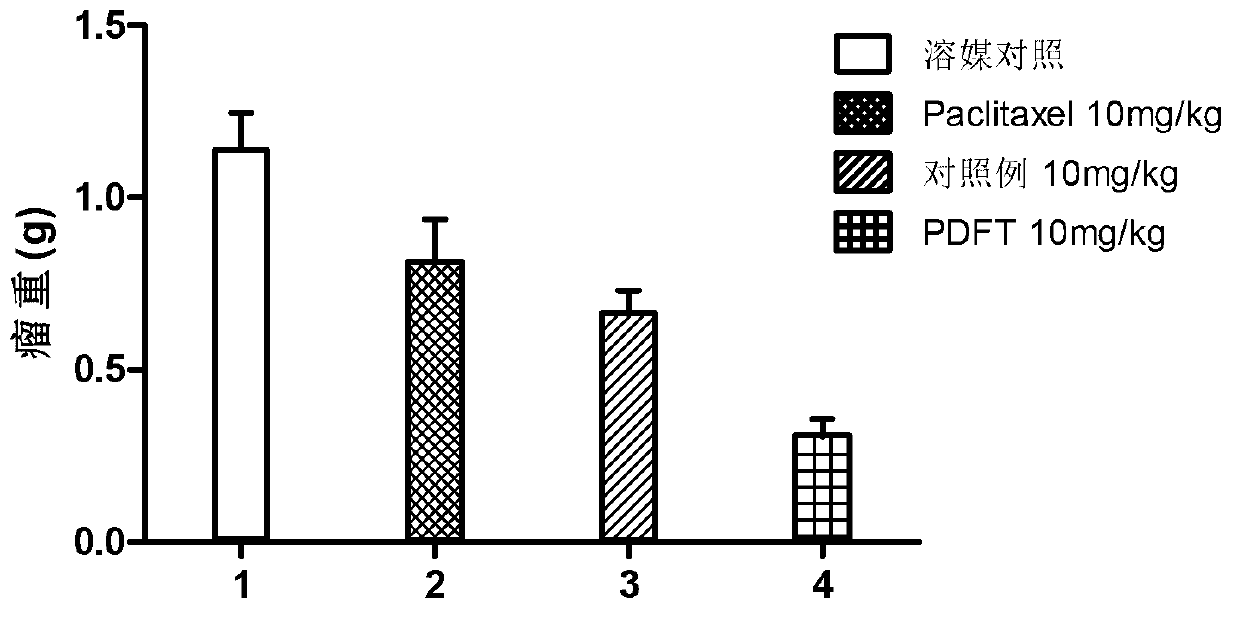
[0063] Polyglutamic acid paclitaxel was dissolved in 0.5 mol / L sodium bicarbonate solution to obtain polyglutamic acid paclitaxel sodium salt. The aqueous solution ...
Embodiment 3
[0072] The preparation of embodiment 3 folic acid-butylene diamine
[0073]
[0074] Dissolve 5 g of folic acid in 20 mL of dimethyl sulfoxide, add 1.66 g of NHS, 4.4 g of EDC, and 0.5 g of DMAP and stir for half an hour. Under vigorous stirring, the solution was slowly added dropwise to a solution of 40 g of butanediamine dissolved in 5 mL of dimethyl sulfoxide, and reacted at room temperature for 12 hours. The reaction solution was rinsed with 1000 mL of dichloromethane, quickly filtered and rinsed under nitrogen protection, and vacuum-dried at 30°C to obtain 5.1 g of a light yellow solid, which was sealed in water-proof form for future use. Yield 87.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com