Nitrogen-doped nano carbon electrocatalyst for fuel cell, and preparation and application of nitrogen-doped nano carbon electrocatalyst
A nitrogen-doped nano-carbon, electrocatalyst technology, applied in battery electrodes, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as no catalytic effect, achieve low cost, change microstructure, and good catalytic activity and stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Preparation of working electrode
[0037] After mixing 5mg of catalyst, 1ml of absolute ethanol, and 50μl of 5% Nafion solution, ultrasonically oscillate evenly, and disperse 10μl of the mixed solution to an area of 0.1256cm 2 The glassy carbon (GC) disk electrode surface was dried to obtain a thin film electrode.
[0038] Stability Accelerated Test Method
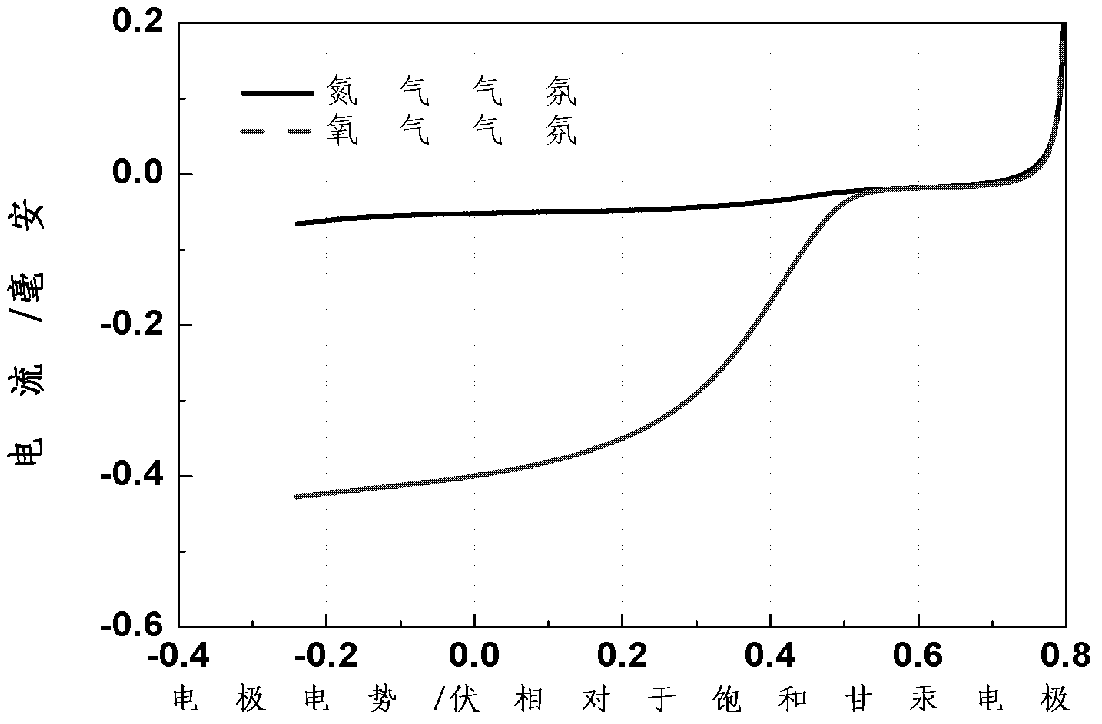
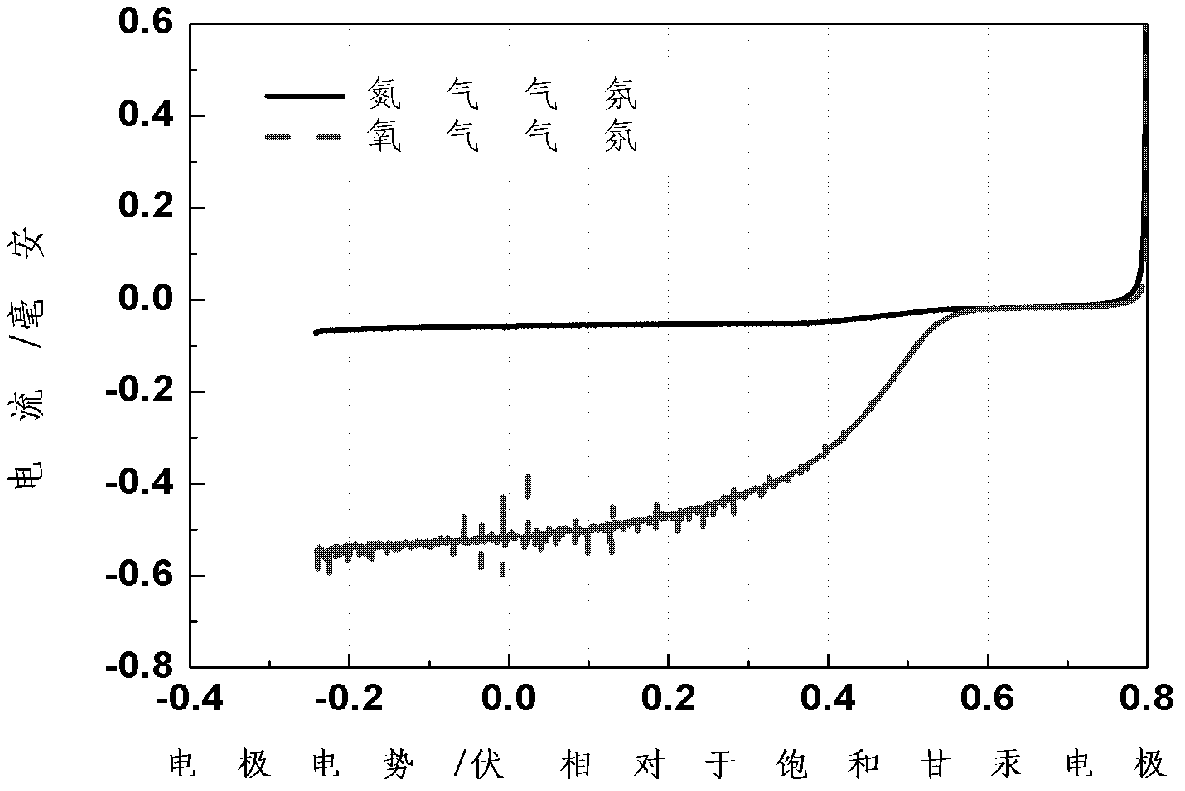
[0039] The stability of the catalyst was evaluated in the following way: the N 2 Dissolve in the electrolyte until the electrolyte is saturated, then cycle CV scan from 0-1.0V vs. SHE (scan rate: 50mV / s, scan number: 1000 times). Before and after the scan, the oxygen reduction polarization curve of the catalyst was tested.
[0040] Test method for oxygen reduction polarization performance in proton exchange membrane fuel cell
[0041] Dissolve oxygen in 0.5M H 2 SO 4 In the electrolyte until the electrolyte is saturated, the current is measured by scanning from the open circuit voltage (OCV) in the negative ...
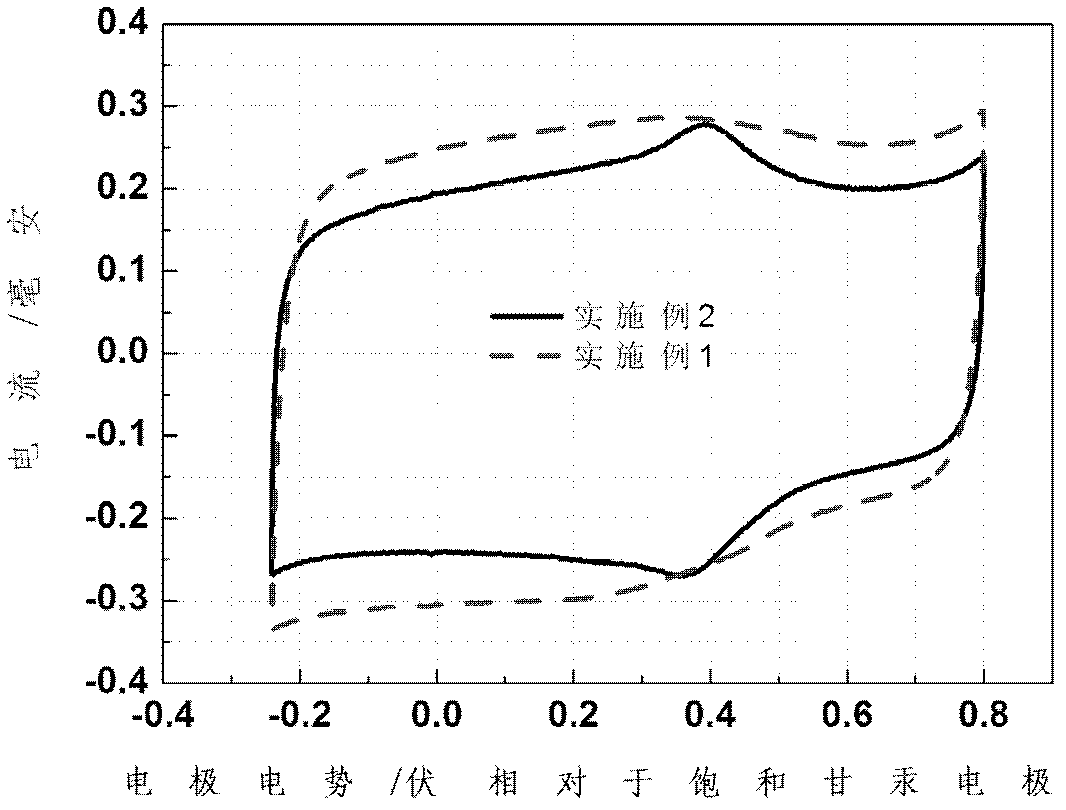
Embodiment 1
[0046] 2.5g of polyvinylpyrrolidone (PVP) was uniformly dispersed in aqueous ethanol (50mL 2 h 5 OH+15mL H 2 O), after stirring for 1 h, add dropwise aniline hydrochloride solution (5mL aniline+3mL 37.5% HCl+14mL H 2 O) 10 mL. After stirring for 30 minutes, 1mol / L HCl solution was added dropwise to adjust the pH of the solution to 3.0. After 1 h of reaction, 25 mL of 0.5 mol / L (NH 4 ) 2 S 2 o 8 The solution was slowly dropped into the above solution, and then 0.36g Co(NO 3 ) 2 ·6H 2 O, after stirring evenly, polymerize at room temperature for 12 hours to obtain a polyaniline solution. Evaporate the polyaniline solution to dryness, wash it, and dry it at 85°C, then treat it in an ammonia atmosphere at 800°C for 3 hours to obtain a black powder; put the above powder into a 1M acidic aqueous solution, treat it with acid for 1 hour, and wash The metal in the catalyst; then it is ball-milled with a ball mill for 3 hours to obtain a doped nano-carbon electrocatalyst with ...
Embodiment 2
[0048] Evenly disperse 2.5g of PVP-K30 in aqueous ethanol (50mL C 2 h 5 OH+15mL H 2 O), stirring
[0049] After 1h, add the now prepared aniline hydrochloride solution (5mL aniline+3mL 37.5% HCl+14mL HCl) dropwise 2 O) 10 mL. After stirring for 30 minutes, 1mol / L HCl solution was added dropwise to adjust the pH of the solution to 3.0. After 1 h of reaction, 25 mL of 0.5 mol / L (NH 4 ) 2 S 2 o 8 The solution was slowly dropped into the above solution, and then 0.35g Fe(NO 3 ) 3 ·6H 2 O, after stirring evenly, polymerize at room temperature for 12 hours to obtain a polyaniline solution. The polyaniline solution was evaporated to dryness at 60°C; after drying at 85°C, it was treated in an ammonia atmosphere at 800°C for 3 hours to obtain a black powder; washing the metals in the catalyst; and then ball milling them with a ball mill for more than 3 hours to obtain a doped nano-carbon electrocatalyst with an ordered nanostructure. The nitrogen content is 6.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com