Reorganizational hemopoietin and preparation method thereof
A technology of erythropoietin and synthetase, which is applied in the field of recombinant erythropoietin and its preparation, and can solve problems such as poor selectivity, incomplete reaction, and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Recombinant erythropoietin with 4-acetylphenylalanyl residue at position 65 and / or position 152 chemically modified by polyethylene glycol
[0041] (1) Obtaining recombinant erythropoietin with 4-acetylphenylalanyl residues at position 65 and / or position 152
[0042] (1a) Construction of recombinant expression vector
[0043] a way:
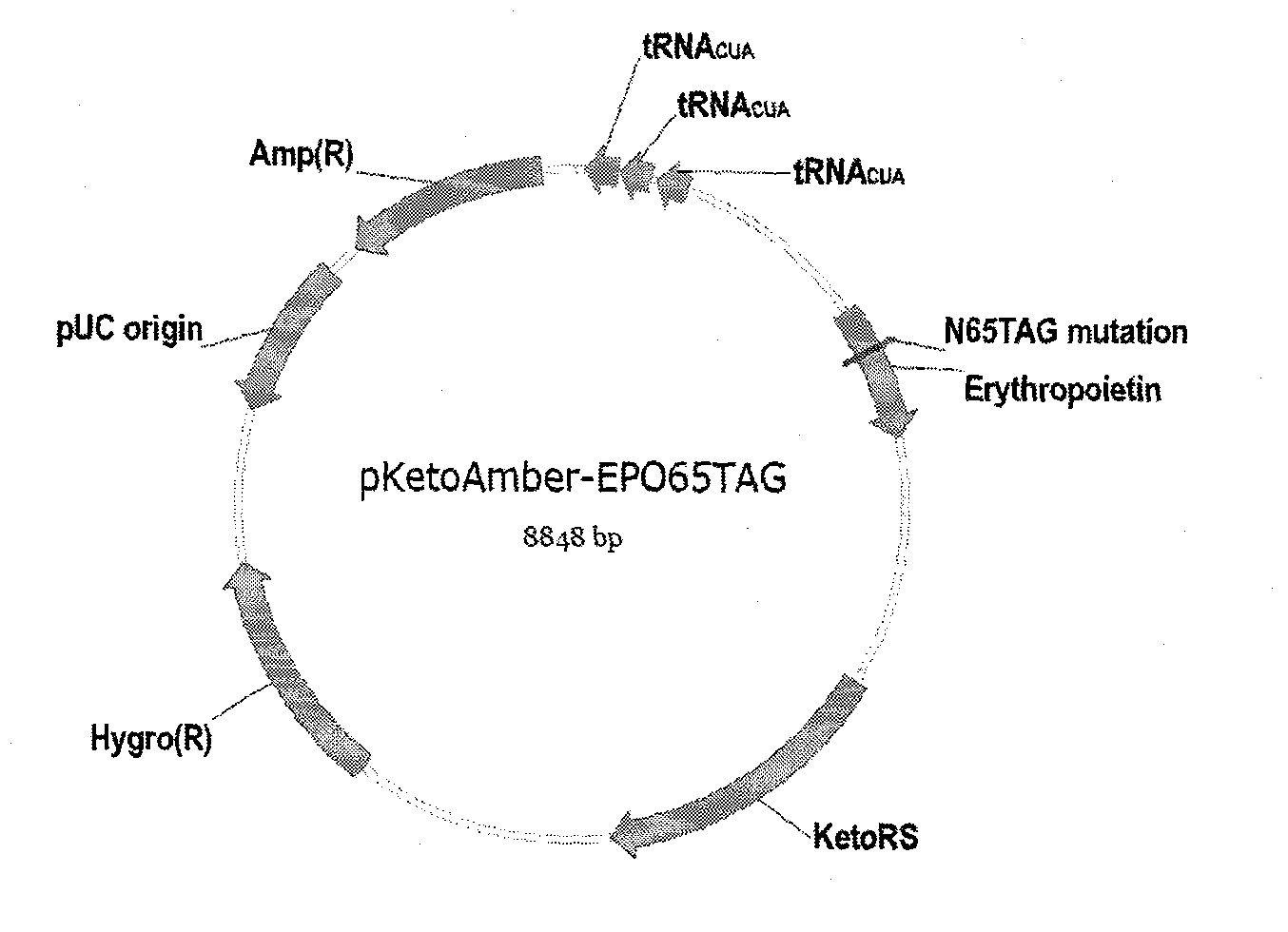
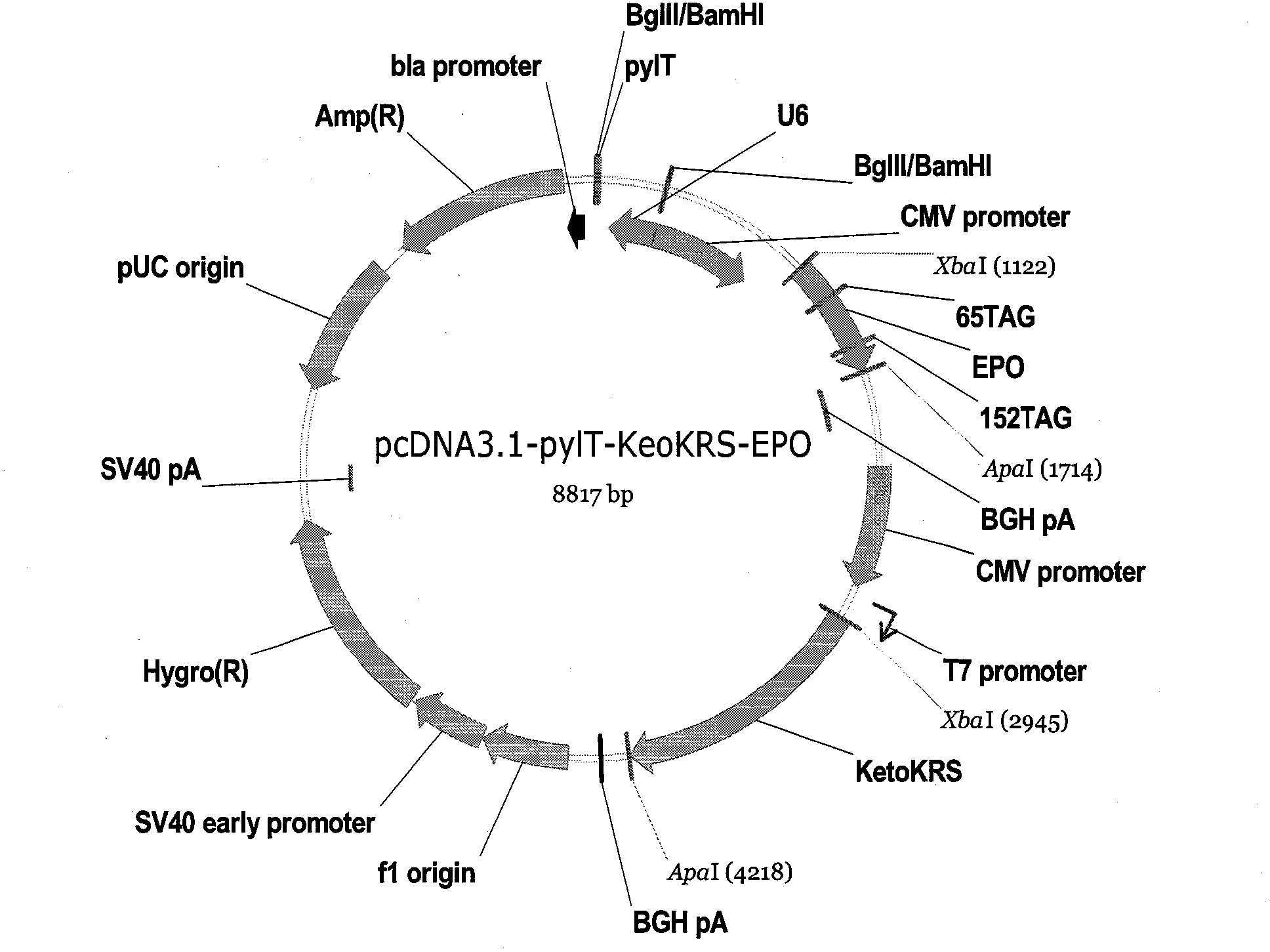
[0044] see figure 1 , at first, construct the expression vector (pKeto) of 4-acetylphenylalanyl-tRNA synthetase gene in vitro, that is: amplify and synthesize the gene that contains coding 4-acetylphenylalanyl-tRNA synthetase (this gene sequence sees The DNA fragment of SEQ ID No.2), the obtained DNA fragment is introduced into a suitable vector by using a specific restriction site to obtain an expression vector containing a gene encoding 4-acetylphenylalanyl-tRNA synthetase. KetoRS (gene sequence is image 3 The DNA fragment of the shown SEQ ID No.2) was separated by agarose electrophoresis, and the gel was recovered to obt...
Embodiment 2
[0075] Example 2: Chemical modification of recombinant erythropoietin with 2-amino-8-oxononanoyl residue at position 65 and / or position 152 with polyethylene glycol
[0076] (1) Obtaining recombinant erythropoietin with 2-amino-8-oxononanoyl residues at position 65 and / or position 152
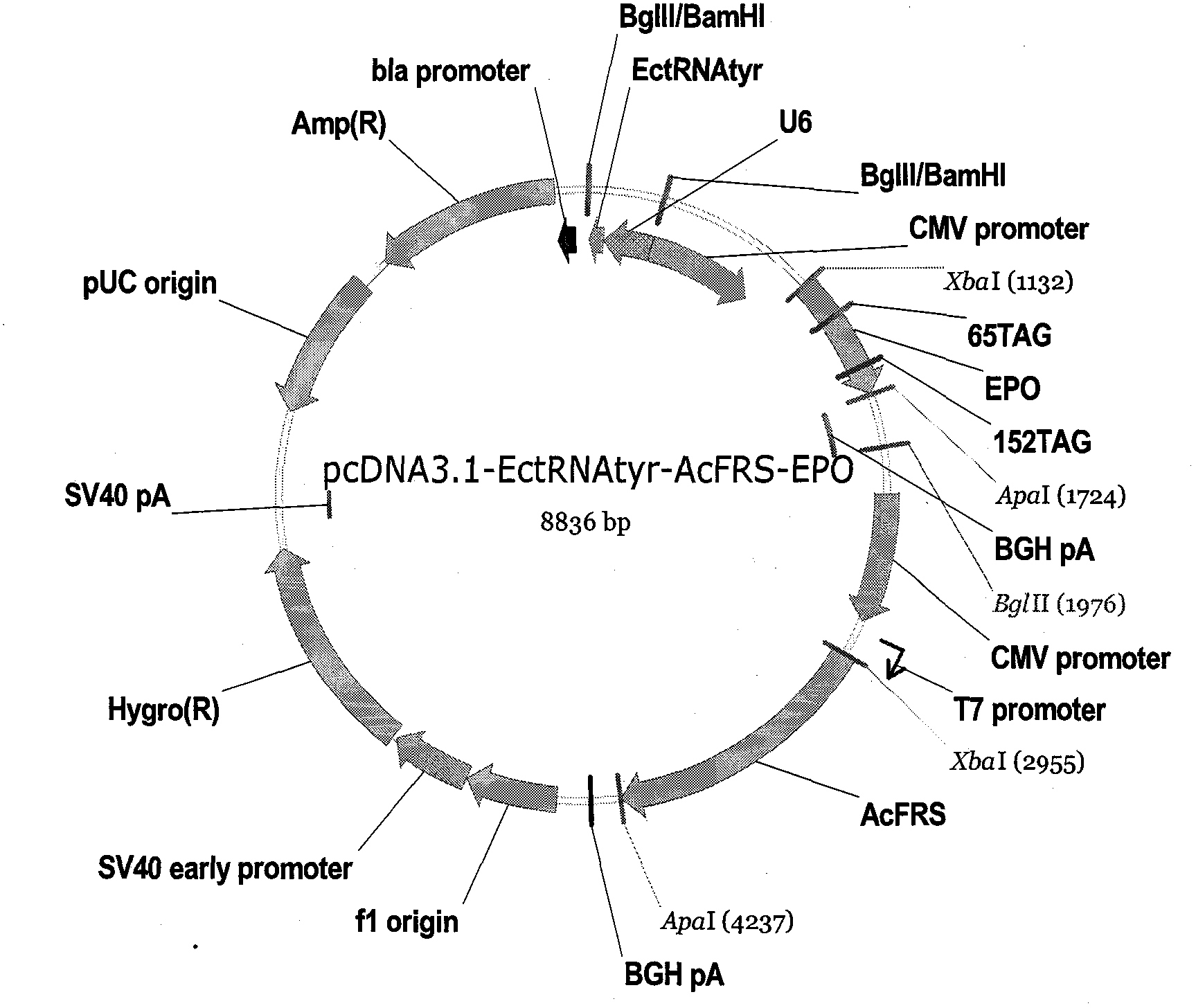
[0077] see image 3 , first, the double helix polynucleotide U6-pylT-BamHI-BglII (sequence is SEQ ID No.17) (ordered from Epoch Biolabs) is digested with endonucleases BamHI and BglII, and then inserted into the plasmid pcDNA3.1 / BglII site of hygro(+), the recombinant plasmid pcDNA3.1-pylT was obtained, and the sequence of PylT was SEQ ID No.5;
[0078] Then, with KetoKRS-XbaI-F (sequence is SEQ ID No.18), KetoKRS-ApaI-R (sequence is SEQ ID No.19) as primer pair 2-amino-8-oxononanoyl-tRNA synthetase ( KetoKRS) (sequence is SEQ ID No.6) to amplify, then digest with endonuclease XbaI, ApaI;
[0079] Then, insert the product digested with endonuclease XbaI and ApaI into the recombinant plasmid...
Embodiment 3
[0085] Embodiment three: the pharmacokinetic research and hematocrit determination of PEGylated EPO (AcF65), EPO (AcF152), EPO (KetoK65) and EPO (KetoK152)
[0086] PEGylated EPO(AcF65), EPO(AcF152), EPO(KetoK65) and EPO( KetoK152) samples with the same concentration. These PEGylated EPO compounds were used to compare pharmacokinetic profiles with native, unmodified, naturally functional EPO [from Amgen, Inc., Thousand Oaks, CA] as a benchmark since it is approved by the FDA. Approved product with long half-life due to protein hyperglycosylation. For the detection of proteins in blood, the chloramine T method, known in the art, was used with 125 I labeled five samples at tyrosine positions. Each molecule has about 1-2 125 I connect. Each sample (80ug) was labeled and desalted from residual unbound 125 I separated, detected the activity of the protein, and verified it with polyacrylamide gel electrophoresis and reversed-phase column. The following subpopulations were ass...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com