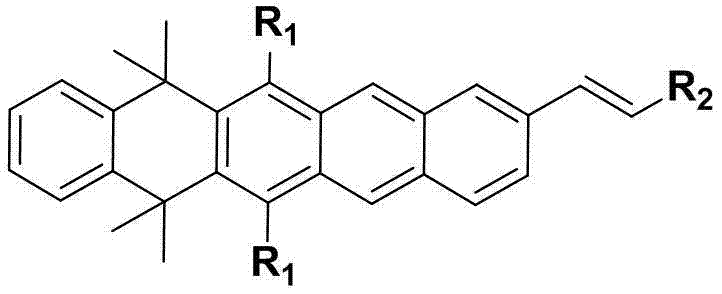
Dihydro-pentacene-olefin organic light-emitting material, preparation method and application of dihydro-pentacene-olefin organic light-emitting material
A technology of luminescent materials and pentaphenylene olefins, applied in the preparation of luminescent materials, organic compounds, organic chemistry, etc., can solve the problems of low luminous efficiency and short lifespan, and achieve the effects of high luminous efficiency, improved performance and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
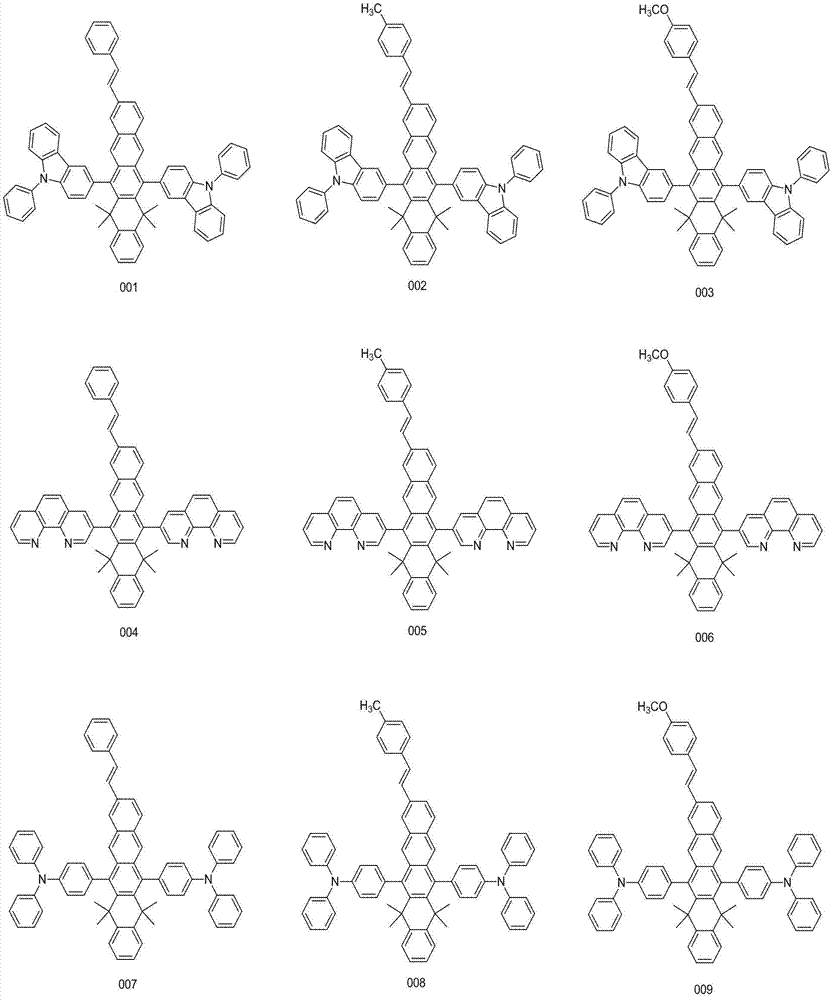
[0033] Embodiment 1: the synthesis of compound 001
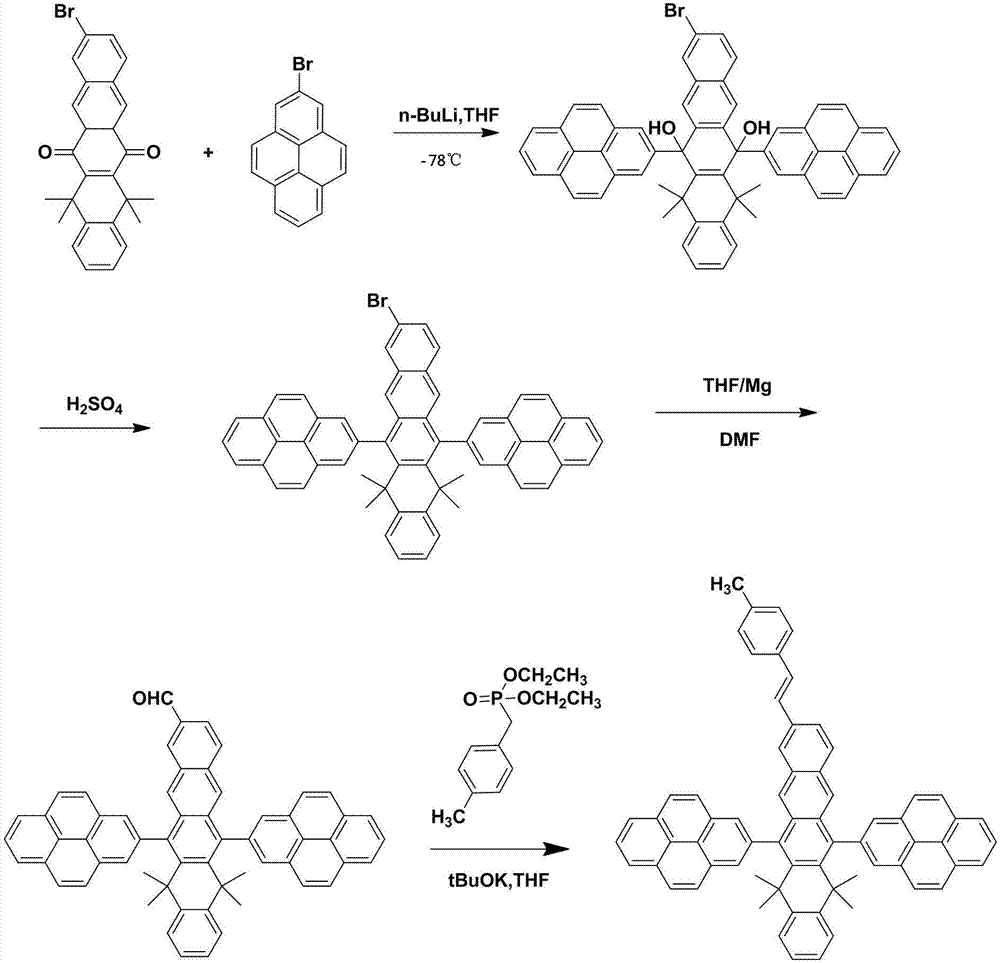
[0034] The specific synthetic route is shown in the following structural formula:
[0035]
[0036](1) Under nitrogen protection system, weigh 110mmol (49.3g) of 3-bromo-N-phenylcarbazole, add 300ml of freshly distilled tetrahydrofuran solution, and add dropwise n-butyllithium 7.19g at -78°C g, reacted for 2 hours, then added dropwise 200ml containing 9-bromo-5,5,14,14-tetramethyl-4a,5,13a,14-dihydropentacene-6,13(4aH,13aH) di Ketone 50mmol (22.4g) tetrahydrofuran solution, after dropwise addition, under the condition of nitrogen protection, react at room temperature for 8 hours, add 100ml of 1M dilute hydrochloric acid, extract 3 times with 100ml of ether, separate liquid, concentrate, use dichloromethane: Petroleum ether=1:3 recrystallized to obtain 41.2g of off-white intermediate N-phenyl-3-carbazolyldihydropentacene alcohol derivatives.
[0037] (2) Take N-phenyl-3-carbazolyldihydropentacene alcohol derivatives, add...
Embodiment 2
[0040] Embodiment 2: the synthesis of compound 004
[0041] The specific synthetic route is shown in the following structural formula:
[0042]
[0043] (1) Under a nitrogen protection system, weigh 110mmol (28.5g) of 3-bromo-phenanthrolinyl, add 300ml of freshly steamed tetrahydrofuran solution, and add 7.19g of n-butyllithium dropwise at -78°C to react 6 hour, then dropwise added 200ml of 9-bromo-5,5,14,14-tetramethyl-4a,5,13a,14-dihydropentacene-6,13(4aH,13aH)dione 50mmol ( 22.4g) of tetrahydrofuran solution, after the dropwise addition, under the condition of nitrogen protection, react at room temperature for 9 hours, add 100ml of 1M dilute hydrochloric acid, extract 3 times with 100ml of ether, separate liquid, concentrate, use dichloromethane:petroleum ether= 1:3 recrystallization to obtain 39.4g off-white intermediate 3-phenanthrolinyldihydropentacene alcohol derivatives.
[0044] (2) Take 3-phenanthrolinyldihydropentacene alcohol derivatives, add them to 300ml of ...
Embodiment 3
[0047] Embodiment 3: the synthesis of compound 007
[0048] The specific synthetic route is shown in the following structural formula:
[0049]
[0050] (1) Under the condition of nitrogen protection, weigh 110mmol (35.5g) of 4-bromo-triarylamino group, add 300ml of freshly steamed tetrahydrofuran solution, and add 7.19g of n-butyllithium dropwise at -78°C to react 4 hours, then dropwise added 200ml containing 9-bromo-5,5,14,14-tetramethyl-4a,5,13a,14-dihydropentacene-6,13(4aH,13aH) dione 50mmol ( 22.4g) of tetrahydrofuran solution, after the dropwise addition, under the condition of nitrogen protection, react at room temperature for 9 hours, add 100ml of 1M dilute hydrochloric acid, extract 3 times with 100ml of ether, separate liquid, concentrate, use dichloromethane:petroleum ether= 1:3 recrystallization to obtain 41.6 g of off-white intermediate triarylaminodihydropentacene alcohol derivatives.
[0051] (2) Take triarylaminodihydropentacene alcohol derivatives, add th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com