Solid superacid bifunctional catalyst and preparation method thereof
A dual-function catalyst, solid super acid technology, applied in chemical instruments and methods, physical/chemical process catalysts, isomerization hydrocarbon production, etc., can solve the problem of no solid super acid found, low equilibrium conversion rate, poor product selectivity, etc. problem, to achieve the effect of small grain size, high equilibrium conversion rate and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
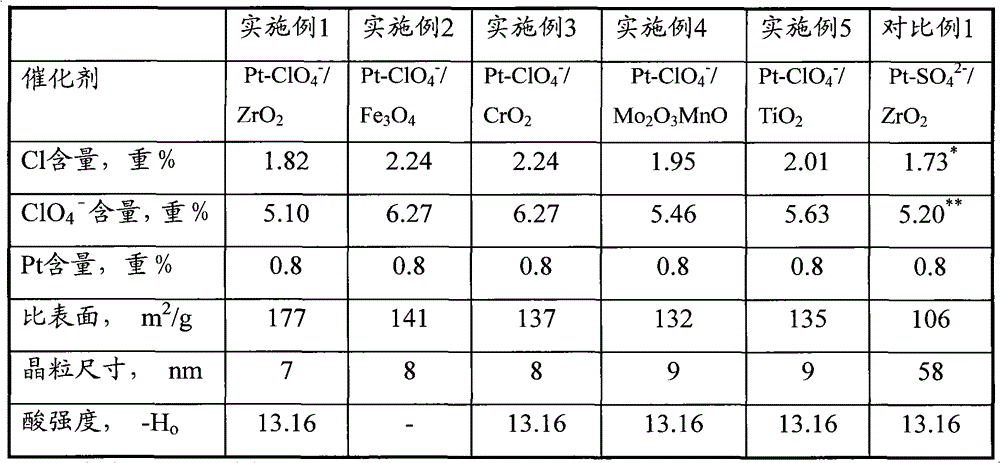
[0051] Embodiment 1 illustrates the Pt-ClO provided by the present invention 4 - / ZrO 2 Catalyst and method for its preparation.
[0052] (1) 108.8gZrOCl 2 ·8H 2 O (Beijing Chemical Plant, analytically pure) was dissolved in 250ml deionized water, and after stirring until the solution was completely clear, it was made into ZrOCl with a concentration of 1.35 mol / liter. 2 solution. in ZrOCl 2 25.0 g H was added to the solution 2 NCONH 2 -Urea (Beijing Yili Fine Chemical Co., Ltd., analytically pure), stirred and dissolved, and the concentration of urea in the solution was 1.67 mol / liter.
[0053] (2) The above solution was poured into a 500ml autoclave, and the temperature was raised to 150° C. under vigorous stirring. At this time, the pH of the gel mother solution was 10.6. The mother liquor was aged at 150° C. under hydrothermal pressure, and the time of the aging process was 45 hours. After the aging, the mother liquor was left to stand for 5 hours, then the supern...
Embodiment 2
[0059] Embodiment 2 illustrates the Pt-ClO provided by the present invention 4 - / Fe 3 o 4 Catalyst and method for its preparation.
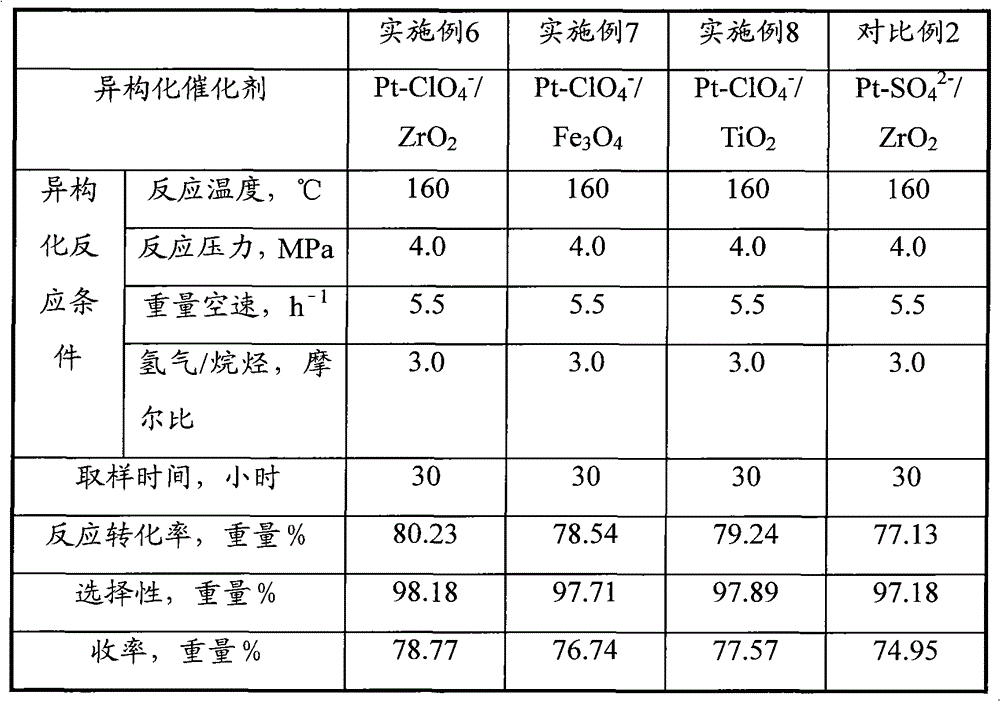
[0060] Catalyst preparation operation step and analysis method of embodiment 2 are identical with embodiment 1, difference is, the metal compound used in step (1) is (FeCl 3 9H 2 (0); the concentration of the ammonium perchlorate solution described in the soaking liquid in the step (4) is 2.5 mol / liter. Obtained Pt-ClO 4 - / Fe 3 o 4 Catalyst, recorded as C-2. Its physical and chemical properties are shown in Table 1.
Embodiment 3
[0062] Embodiment 3 illustrates the Pt-ClO provided by the present invention 4 - / CrO 2 Catalyst and method for its preparation.
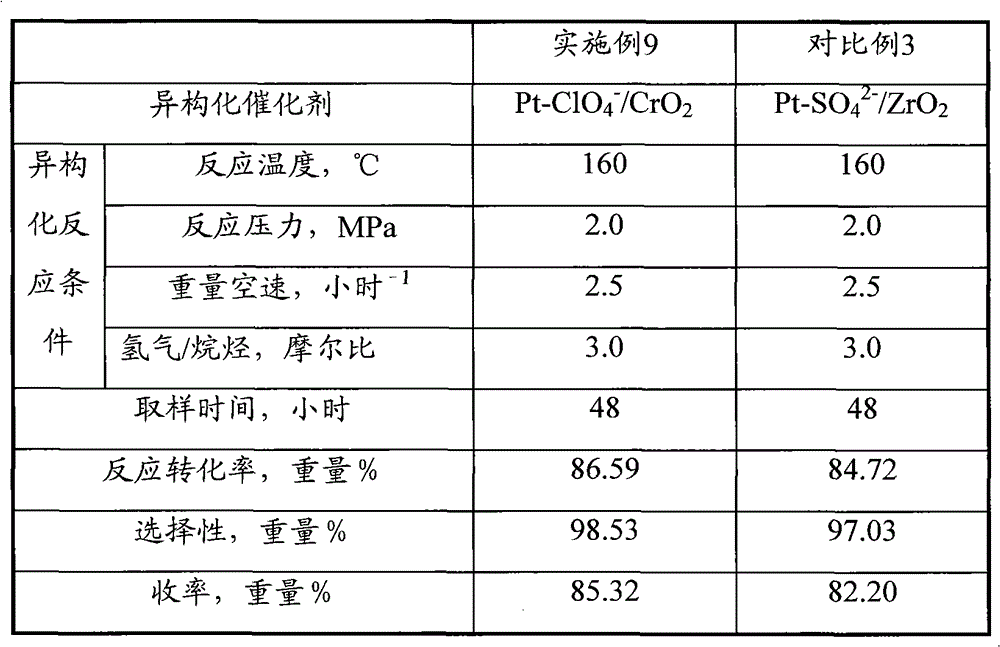
[0063] Catalyst preparation operation step and analysis method of embodiment 3 are identical with embodiment 1, difference is, metal compound used in step (1) is 40gCrCl 3 9H 2 O; The concentration of the ammonium perchlorate solution described in the soaking liquid in the step (4) is 2.5 mol / liter. Obtained Pt-ClO 4 - / CrO 2 Catalyst, recorded as C-3. Its physical and chemical properties are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com