Citrin immunodeficiency disease virulence gene SLC25A13 high frequency mutation screening reagent box
A disease-causing gene and mutation screening technology, applied in the direction of recombinant DNA technology, DNA / RNA fragments, microbial determination / inspection, etc., can solve the problems of time-consuming and expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
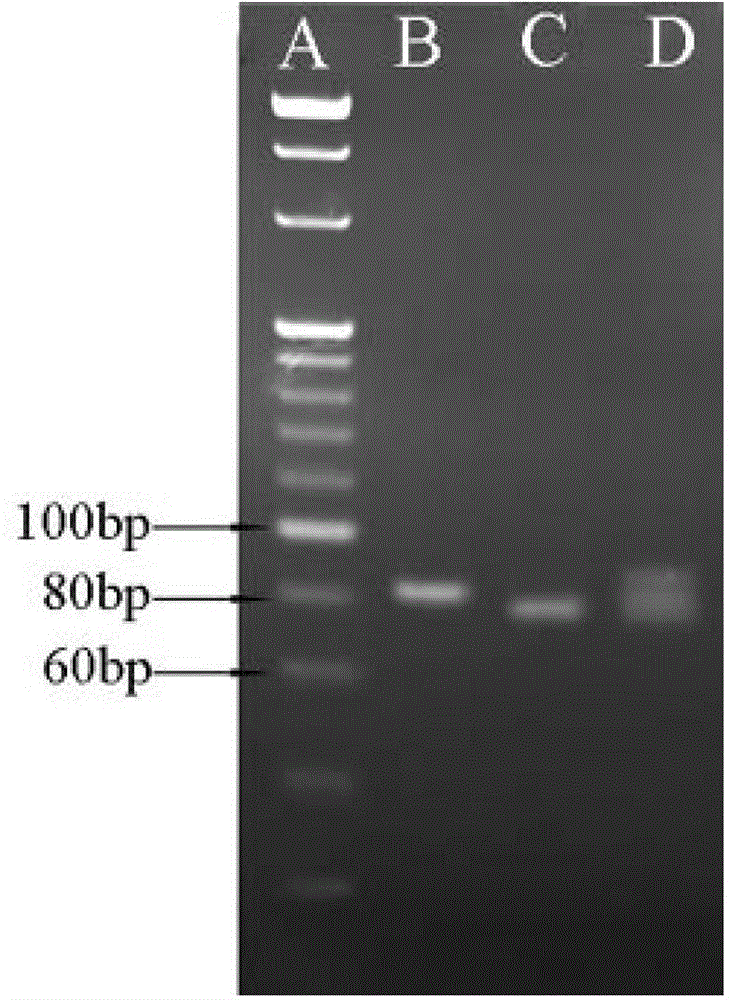
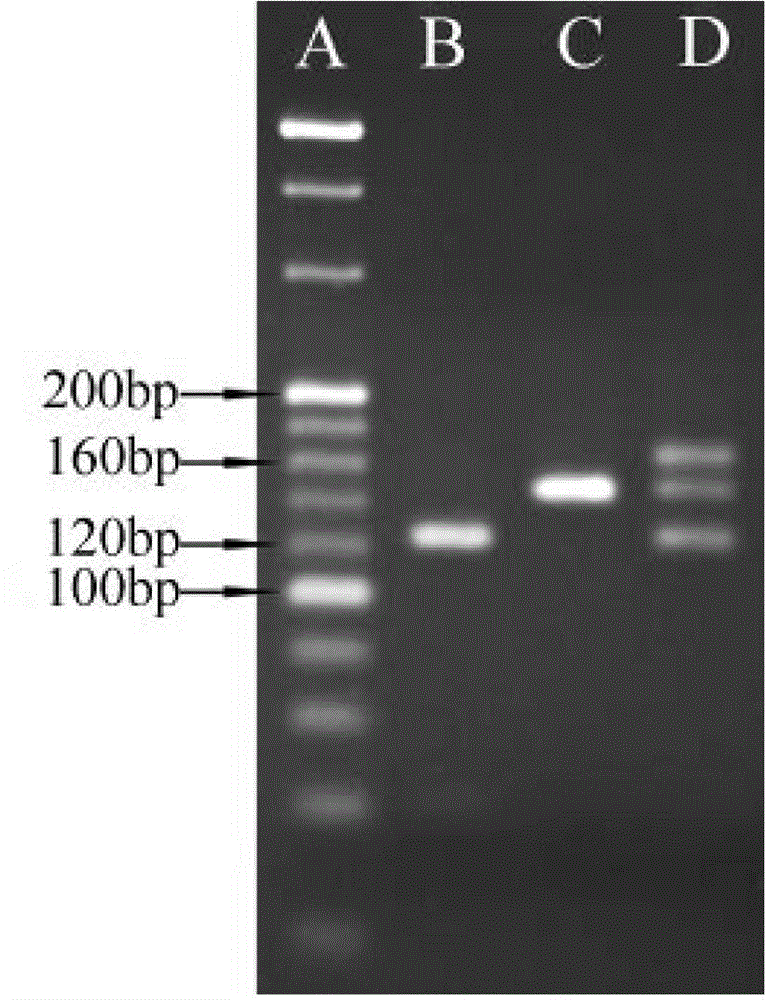
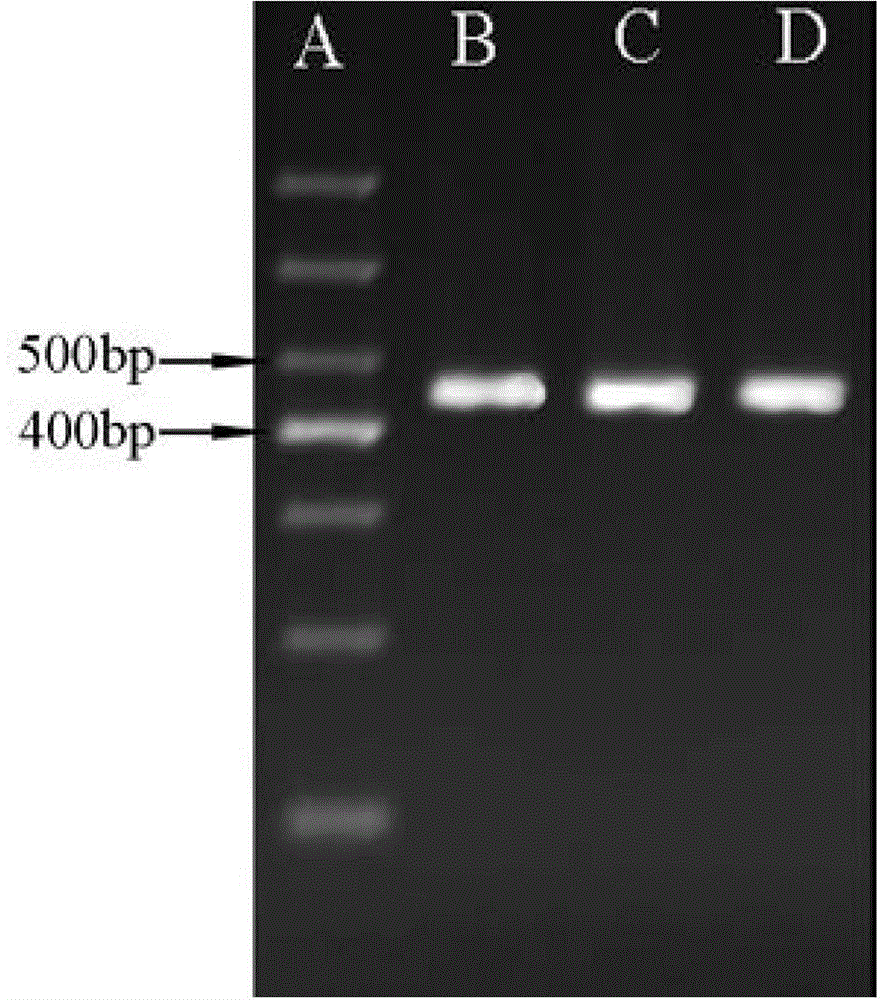
[0031] (1) Extraction of sample genomic DNA
[0032] Patients with four known mutation types of Citrin deficiency, type I, type III, type X and type XIX (each mutation type includes one case of mutation homozygosity and one case of mutation heterozygosity) and normal people without Citrin deficiency disease (normal control) 400 μL of peripheral anticoagulated blood samples were used to extract the genomic DNA of the samples using Simgen’s Whole Blood DNA Mini Kit according to its instructions to obtain genomic DNA at a concentration of 50-150 ng / μL.
[0033] (2) Detection of SLC25A13 type I, type III and type X mutations
[0034] 1) Using the genomic DNA extracted in (1) as a template, carry out PCR amplification, the primers used are shown in Table 1, and the DNA polymerase is rTaq enzyme of TaKaRa.
[0035] Table 1 Four kinds of SLC25A13 mutation detection primers and amplified fragment length
[0036]
[0037] 50μL PCR reaction system: DNA template (50-150ng / μL) 1μL, r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com