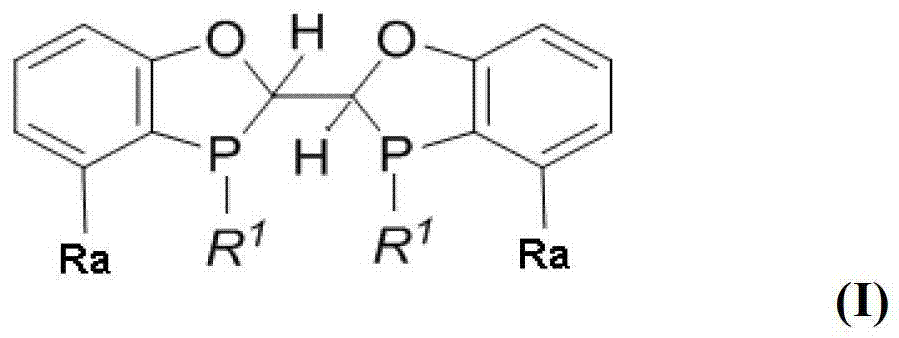
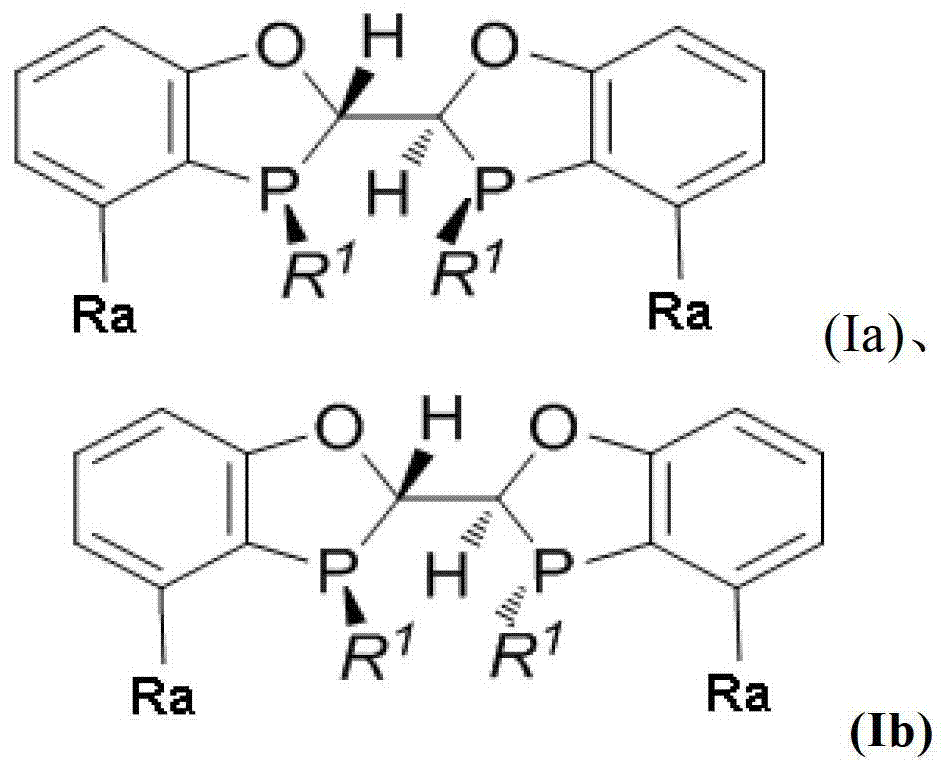
Chiral phosphine ligand and metal catalyst comprising same and application of chiral phosphine ligand and metal catalyst
A technology of bidentate phosphine ligands and transition metals, which is applied in the application field of chiral phosphine ligands and efficient catalytic hydrogenation of chiral β-arylamides, which can solve the problems of difficult substrate synthesis and insufficient efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
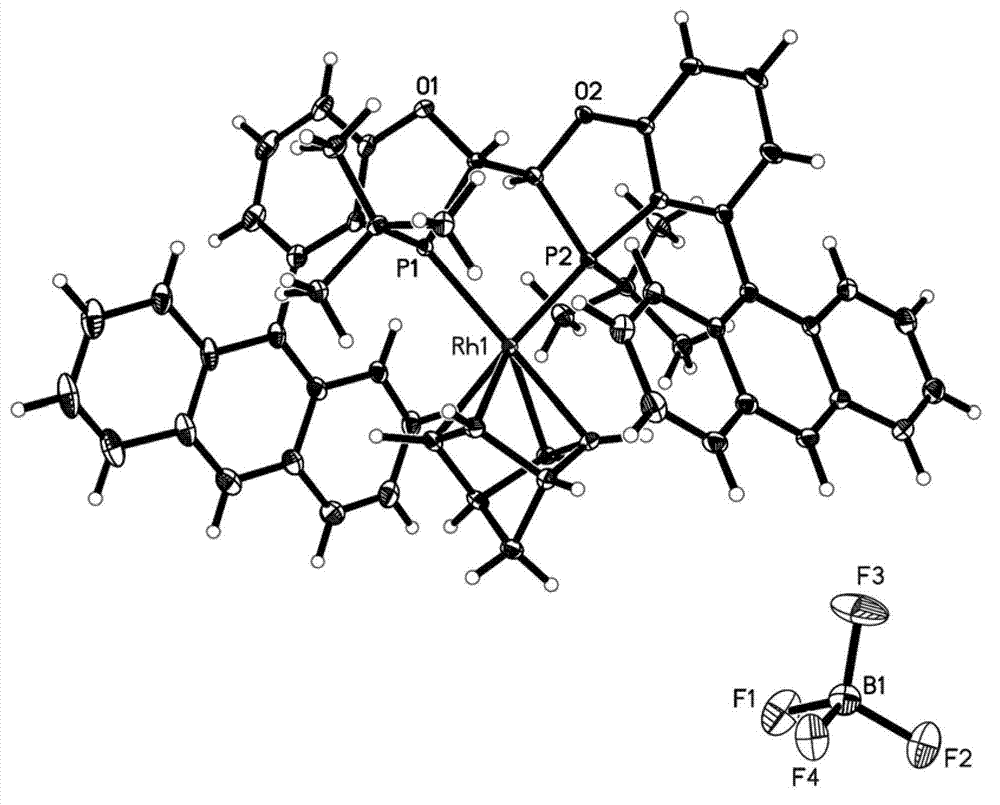
[0125] In this example, (2S,2'S,3S,3'S)-4,4'-di(9-anthracenyl)-3,3'-di-tert-butyl-2,2',3,3'-tetrahydro -2,2'-dibenzo[d][1,3]oxy,phosphorus-pentaconjugate (1) and its metal complex {(norbornadiene)[(2S,2'S,3S,3'S)- 4,4'-bis(9-anthracenyl)-3,3'-di-tert-butyl-2,2',3,3'-tetrahydro-2,2'-dibenzo[d][1, 3] Oxygen,phosphorus-pentyl yoke} rhodium tetrafluoroborate, namely Rh(nbd)(1)BF 4 The preparation (its reaction scheme as shown below) is an example to describe the preparation method of chiral bisphosphine ligand of the present invention and metal rhodium complex thereof in detail:
[0126]
[0127] 1. (S)-4-(9-anthryl)-3-tert-butyl-2,3-dihydrobenzo[d][1,3]oxo,phospho-pentyl-3-oxo(c) preparation of
[0128] Following known literature procedures, a was converted to (S)-4-(9-anthryl)-3-(tert-butyl)-2,3-dihydrobenzo[d][1,3]oxo, Phosphorus-pentyl-3-oxo (c,Org.Lett.2011,13,1366)
[0129] 2. (2S,2'S,3R,3'R)-4,4'-bis(9-anthracenyl)-3,3'-di-tert-butyl-2,2',3,3'-tetrahydro- Preparati...
Embodiment 2
[0139] This example takes the preparation of substrate 13a (its reaction scheme is shown below) as an example to describe the preparation method of (E)-β-aryl enamide of the present invention in detail:
[0140]
[0141] 1. Preparation of (E)-1,3-dimethoxy-5-(2-nitro-1-propenyl)benzene (f)
[0142] To a 50 mL round bottom flask was added 3,5-dimethoxybenzaldehyde (e, 3.32 g, 20 mmol), ammonium acetate (1 g, 26 mmol), nitroethane (30 mL). After stirring under reflux for 2 hours, it was concentrated, and the residue was dissolved in 50 mL of dichloromethane, and washed with water and saturated brine (50 mL) successively. After drying over anhydrous sodium sulfate, concentrate to obtain the crude target product (E)-1,3-dimethoxy-5-(2-nitro-1-propenyl)benzene (f, 4.24g, 95% yield rate), the product was directly used in the next reaction.
[0143] 2. Preparation of (E)-1-(3,5-dimethoxyphenyl)-2-acetamidopropene (13a)
[0144] Under nitrogen protection, add (E)-1,3-dimethoxy-5...
Embodiment 3
[0147] In this example, the preparation of 2-acetamido-3,4-dihydronaphthalene (13q) (the reaction scheme is shown below) is taken as an example to illustrate the cyclic β-aryl-N-acetylene described in the present invention The preparation method of amine:
[0148]
[0149] Preparation of 2-acetamido-3,4-dihydronaphthalene (13q)
[0150] Under nitrogen protection, add tetralone (1.46g, 10mmol, 1 equivalent), acetamide (1.83g, 25mmol, 2.5 equivalents), monohydrate-p-toluenesulfonic acid (0.19g, 1mmol , 0.1 eq), toluene (60 mL). Put on the water separator, reflux and stir for 20 hours, then cool down to room temperature. 150 mL of saturated aqueous sodium bicarbonate was added for washing, and the aqueous phase was extracted twice with 100 mL of ethyl acetate. The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and the organic phase was concentrated. The crude product was subjected to silica gel column chromatography (petroleu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com