New method for generating methyl ketone by using palladium catalytic oxidized olefins
A technology for oxidizing alkenes and methyl ketones, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of difficult laboratory preparation and industrial application, expensive palladium ligands, harsh reaction conditions, etc. Achieve the effects of high substrate conversion rate and product yield, fast reaction speed and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
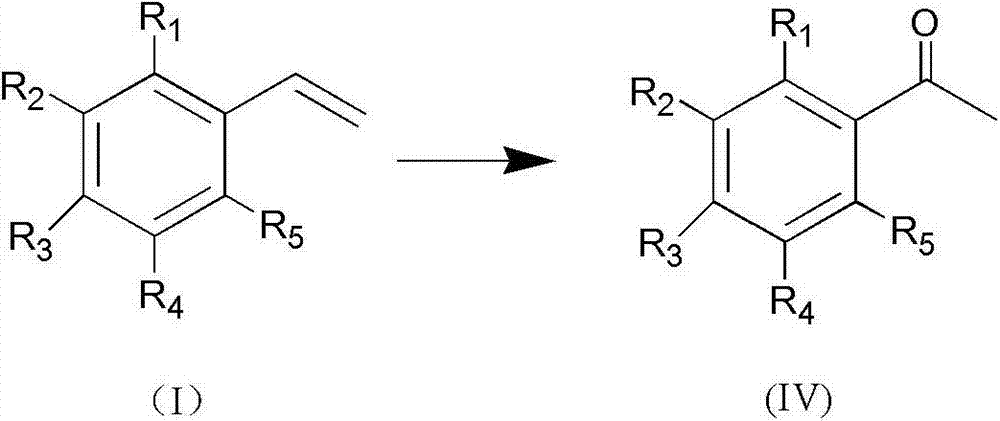
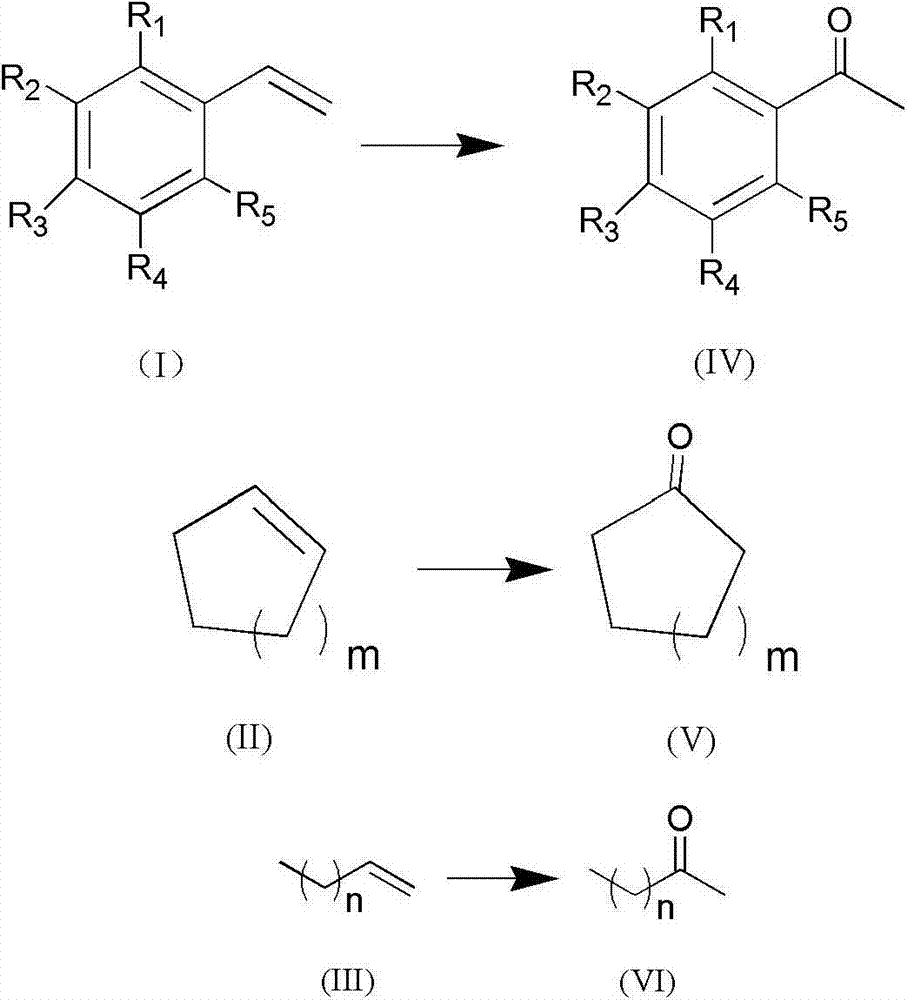
[0033] In a 100mL Schlenk tube equipped with magnetic stirring, the whole system is in an all-oxygen atmosphere by repeatedly pumping oxygen, and then adding p-methylstyrene (that is, R in structural formula (I) 3 is methyl, R 1 , R 2 , R 4 , R 5 All hydrogen) 1mmol, palladium acetate 0.02mmol, benzoquinone 0.1mmol, sodium nitrite 0.2mmol, quickly add 5.0mL solvent (methanol: water = 4:0.5 (v / v)) and perchloric acid 0.4mmol, and then immediately The whole system was sealed in an oxygen atmosphere, and reacted at room temperature for 5 hours. After the reaction was completed, the solution was rotary evaporated, and the residue was purified with a silica gel column (eluent, petroleum ether: ethyl acetate = 10:1) to obtain the target product pair Methyl acetophenone, the yield is 91%. 1 H NMR (500MHz, CDCl 3 ):δ2.62(s,3H),7.47(t,J=7.5Hz,2H),7.58(t,J=7.0Hz,1H),7.97(t,J=4.5Hz,2H). 13 C NMR (125MHz, CDCl 3 ): δ26.5, 128.2, 128.5, 133.0, 137.1, 198.1.
Embodiment 2
[0035] The reactant used is p-bromostyrene (i.e. R in the structural formula (I) 3 is bromine, R 1 , R 2 , R 4 , R 5 Both are hydrogen), the experimental method and steps are the same as in Example 1, add solvent 5.0mL (acetonitrile: water = 5:0.5 (v / v)) and hydrochloric acid 0.3mmol, catalyst palladium acetate consumption is 0.05mmol, benzoquinone 0.1mmol, Sodium nitrite 0.2mmol, the reaction temperature is 30°C, the reaction time is 24h, and the yield is 85%. 1H NMR (500MHz, CDCl 3 ):δ2.60(s,3H),7.61-7.63(q,2H),7.83-7.84(q,2H). 13 C NMR (125MHz, CDCl 3 ): δ26.5, 128.4, 129.8, 131.9, 135.8, 197.1.
Embodiment 3
[0037] The reactant used is o-chlorostyrene (i.e. R in the structural formula (I) 1 is chlorine, R 3 , R 2 , R 4 , R 5 Both are hydrogen), the experimental method and steps are the same as in Example 1, add solvent 5.0mL (dichloromethane: water = 5:0.5 (v / v)) and acetic acid 0.3mmol, catalyst palladium acetate consumption is 0.05mmol, benzoquinone 0.1 mmol, 0.2 mmol of sodium nitrite, the reaction temperature is 25° C., the reaction time is 5 h, and the yield is 87%. 1 H NMR (500MHz, CDCl 3 ): δ2.64(s,3H),7.28-7.33(q,1H),7.36-7.42(q,2H),7.53-7.55(q,1H). 13 C NMR (125MHz, CDCl 3 ): δ30.7, 126.9, 129.4, 130.6, 131.3, 132.0, 139.1, 200.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com