Alkyl-substituted quinolinone compound with side chains as well as preparation method, pharmaceutical composition and application thereof
The technology of a compound, quinolinone, is applied in the field of medicine to achieve the effects of enhanced proton pump inhibition, strong anti-Helicobacter pylori effect, and overcoming technical defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
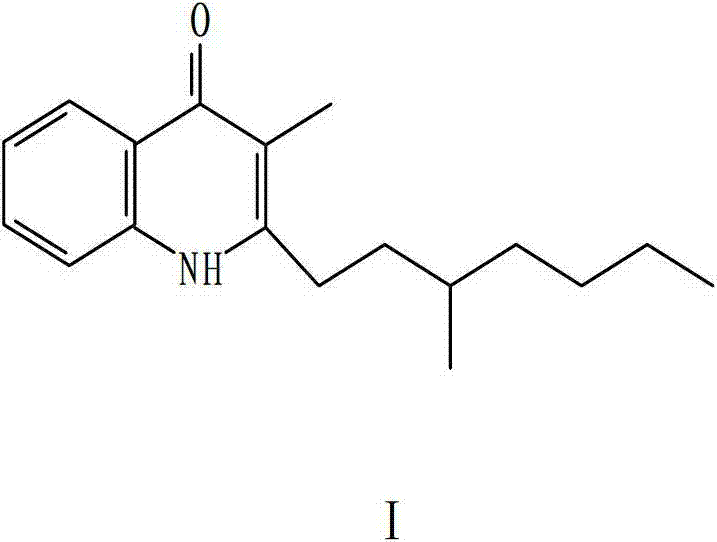
[0043] Embodiment 1, preparation synthesis heptanolinone compound:
[0044] 1. Take 13mmol of o-aminopropiophenone and 20mmol of 4-methyl-octanoyl chloride, put them in a 250ml double-neck round bottom flask, add 18mmol of pyridine, 60-80ml of dichloromethane, stir and react at room temperature for 4-20 hours, and add saturation Ammonium chloride solution 60-100ml, take the organic phase, concentrate under reduced pressure, conduct silica gel column chromatography on the residue, collect the target object, concentrate under reduced pressure, and dry to obtain 4-methyl-octylaminopropiophenone;
[0045] 2. Put 11mmol of 4-methyl-octanoylaminopropiophenone in step (1) into a 250ml double-neck round bottom flask, add 30-40mmol of sodium tert-butyl alkoxide, 180-240ml of anhydrous organic solvent, and reflux for 4- After 36 hours, concentrate under reduced pressure to recover ethanol, add 100-200ml of water to elute once, and the residue is subjected to silica gel column chromatog...
Embodiment 2
[0049] Embodiment 2, the method for preparing heptanolinone tablet:
[0050] Taking the heptanolinone compound prepared in Example 1 as raw material, prepare heptanolinone tablet, the formula of 1000 tablets is as follows:
[0051] Heptanolone (as C 18 h 22 NO meter)
[0052] Mix the raw materials and auxiliary materials, grind them into fine powder, pass through a 100-mesh sieve, take the amount according to the formula, press into tablets, and pack.
Embodiment 3
[0053] Embodiment 3, the method for preparing heptanolone capsule:
[0054] Taking the heptanolinone compound prepared in Example 1 as raw material, prepare heptanolinone capsules, the formula of 1000 capsules is as follows:
[0055] Heptanolone (as C 18 h 22 NO meter)
Starch and other accessories
100
150
[0056] Mix the raw materials and auxiliary materials, grind them into fine powder, pass through a 100-mesh sieve, take the amount according to the formula, granulate and pack.
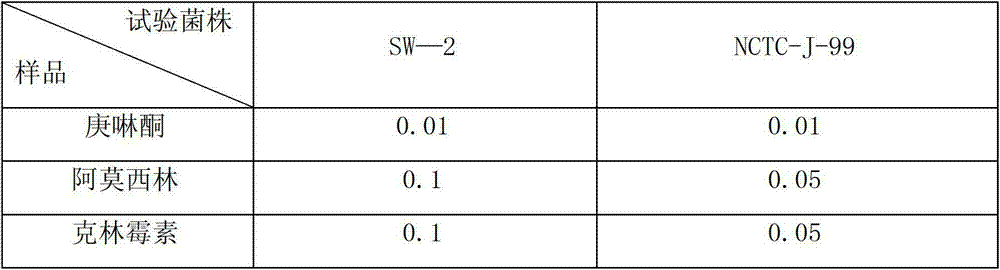
[0057] Biological test 1: Antibacterial effect of heptanolone:
[0058] According to the literature (Ibrahim M, Khan AA, Tiwari SK, et a1. Antimierobia activity of Sapindus mukorossi and Rheum emodi extracts against H. priori: in vitro and in vivo studies. World J Gastroenterol, 2006.14: 7136-7142.) method The effect of heptanolone against clinically isolated Helicobacter pylori SW-2 and standard strains was tested (Table 1). Linmycin.
[0059] Table 1 MIC ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com