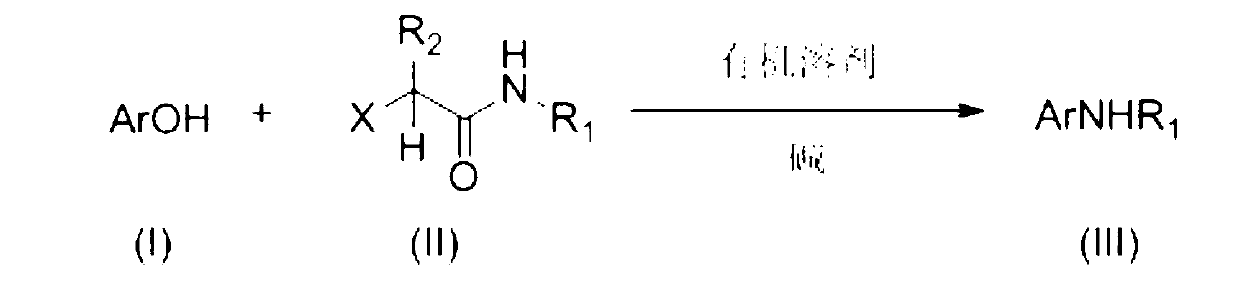
Method preparing N-aryl and N-alkyl aromatic amine type compound from phenol type compound
A technology for phenolic compounds and alkylarylamines is applied in the field of preparing N-aryl and N-alkylarylamine compounds and achieves the effects of mild reaction conditions, low cost and pollution avoidance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the synthesis of diphenylamine
[0027] Add phenol (94 mg, 1.0 mmol), KOH (168 mg, 3.0 mmol), 2-chloro-N-phenylpropanamide (549 mg, 3.0 mmol) and toluene (3 ml) to the dry reaction tube, 50 ℃ for 2 hours, add KOH (168 mg, 3.0 mmol), react at 110 ℃ for 24 hours, add 10 ml of water, extract with dichloromethane (3×25ml), and use saturated brine (2×20 ml) for the organic phase ), washed with anhydrous sodium sulfate, filtered, rotary evaporated, and flash silica gel column chromatography (eluent: petroleum ether / ethyl acetate=20 / 1), the product diphenylamine was obtained as a white solid with a yield of 83%.
[0028] 1 H NMR (400 MHz, CDCl 3 , TMS) δ 7.29 (t, J = 8.0 Hz, 4H), 7.09 (d, J = 7.6 Hz, 4H), 6.95 (t, J = 7.6 Hz, 2H), 5.72 (bs, 1H); 13 C NMR (100 MHz, CDCl 3 , TMS) δ 143.0, 129.3, 120.9, 117.8 ppm.
Embodiment 2
[0029] Embodiment 2: the synthesis of 4-chlorodiphenylamine
[0030] Add p-chlorophenol (128 mg, 1.0 mmol), NaOH (80 mg, 2.0 mmol), 2-bromo-N-phenylpropanamide (227 mg, 1.0 mmol) and N-methylpyrrolidone to a dry reaction tube (3 ml), react at 60°C for 4 hours, add NaOH (40 mg, 1.0 mmol), react at 130°C for 2 hours, add 10 ml of water, extract with dichloromethane (3 × 25ml), and use saturated salt for the organic phase Wash with water (2×20 ml), dry over anhydrous sodium sulfate, filter, rotary evaporate, and flash silica gel column chromatography (eluent: petroleum ether / ethyl acetate=20 / 1) to obtain the product 4-chlorodiphenylamine, White solid, yield 75%.
[0031] 1 H NMR (400 MHz, CDCl 3 , TMS) δ 7.32-7.22 (m, 4H), 7.08-6.96 (m, 5H), 5.71(bs, 1H); 13 C NMR (100 MHz, CDCl 3 , TMS) δ 142.5, 141.8, 129.3, 129.1, 125.4, 121.4, 118.7, 118.0 ppm.
Embodiment 3
[0032] Embodiment 3: the synthesis of 3-methoxydiphenylamine
[0033] Add 3-methoxyphenol (124 mg, 1.0 mmol), Na 2 CO 3 (212 mg, 2.0 mmol), 2-iodo-N-phenylpropanamide (522 mg, 2.0 mmol) and N, N-dimethylformamide (3 ml), reacted at 70 ° C for 3 hours, added Na 2 CO 3 (212 mg, 2.0 mmol), reacted at 140 °C for 16 hours, added 10 ml of water, extracted with dichloromethane (3 × 25 ml), washed the organic phase with saturated brine (2 × 20 ml), dried over anhydrous sodium sulfate, and filtered , rotary evaporation, and flash silica gel column chromatography (eluent: petroleum ether / ethyl acetate=20 / 1), the product 3-methoxydiphenylamine was obtained as a light yellow solid with a yield of 48%.
[0034] 1 H NMR (400 MHz, CDCl 3 , TMS) δ 7.29 (t, J = 8.0 Hz, 2H), 7.18 (t, J = 8.0 Hz, 1H), 7.10 (d, J = 7.6 Hz, 2H), 6.96 (t, J = 7.6 Hz, 1H ), 6.67-6.65 (m, 2H), 6.49 (d, J = 8.8Hz, 1H), 5.72 (s, 1H), 3.79 (s, 3H); 13 C NMR (100 MHz, CDCl 3 , TMS) δ 160.6, 144.4, 142.6, 130.0 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com