Preparing method of high-purity polymyxin B
A polymyxin, high-purity technology, applied in the field of medicine, can solve the problems such as difficult to remove B3 and B1-1, and achieve the effect of high purity, high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
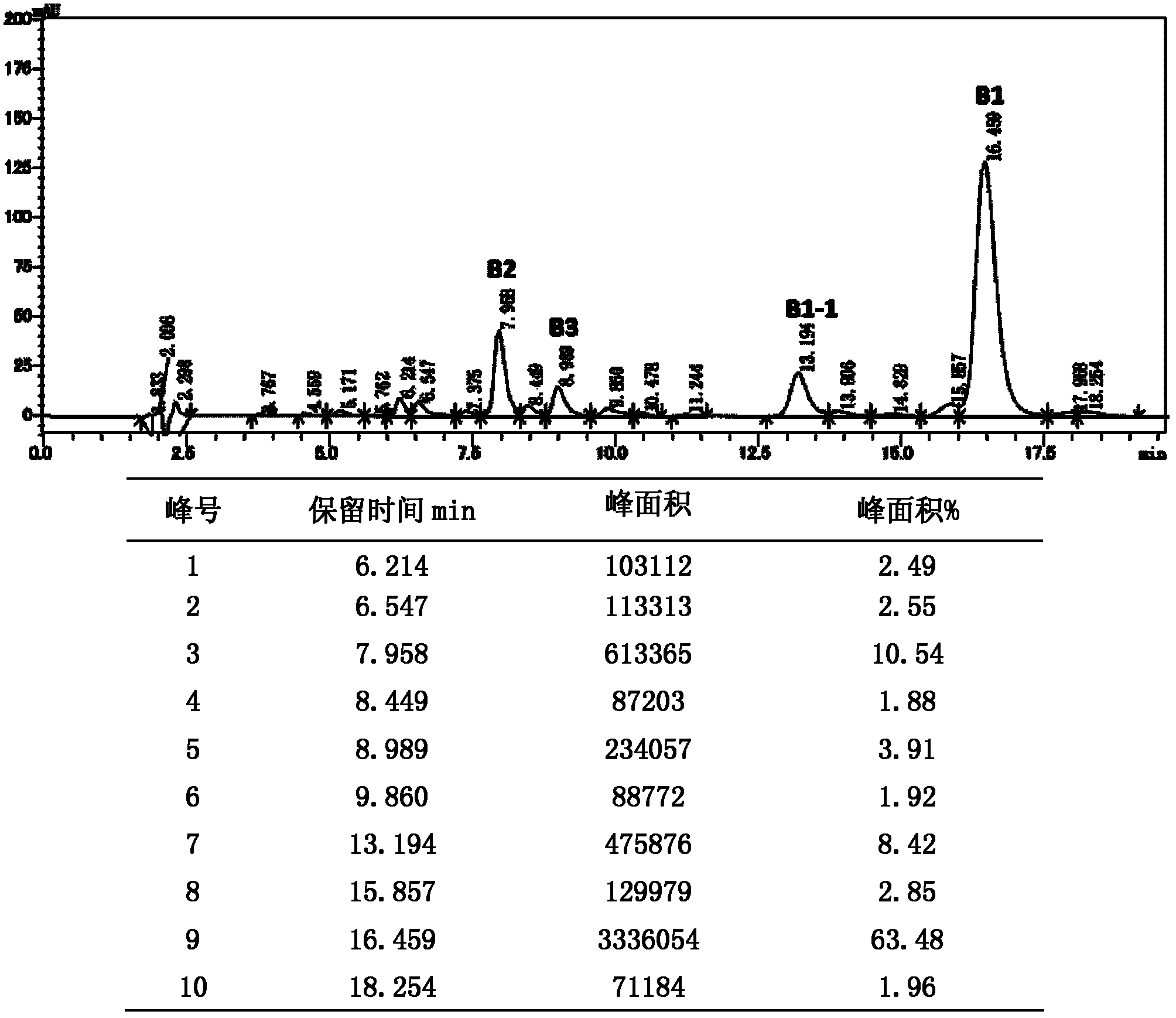
[0028] (1) Preparation of upper column liquid: 1 g of polymyxin B sulfate (the chromatographic purity of polymyxin B1+polymyxin B2 is 74.0%) is dissolved in 100 ml of water through fermentation and separation and extraction of raw material polymyxin B. Filter through a microporous 0.45 μm filter membrane to remove impurities to obtain a clear solution; sample the clear solution for HPLC detection, see chromatographic purity figure 1 , impurity B3 is 3.9%, impurity B1-1 is 8.4%;
[0029] (2) upper column separation: the mobile phase is methanol and sodium sulfate-phosphate buffer solution with a volume ratio of 15:85, and the sodium sulfate-phosphate buffer solution is an aqueous sodium sulfate solution of 20 mM adjusted to pH=2.1 with phosphoric acid; (1) The obtained clarified solution is injected into a preparative HPLC column with a flow rate of 1.0ml / min. The HPLC preparative column is Kromasil 100-3.5-C18 (250 × 4.6mm), and the particle diameter of filler C18 is 5 μm. Th...
Embodiment 2
[0032] (1) Preparation of the upper column liquid: the polymyxin B sulfate raw material is the same as in Example 1, 1.0 g of the sample is taken, dissolved in 25 ml of water, and filtered through a 0.45 μm filter membrane to obtain a clear solution;
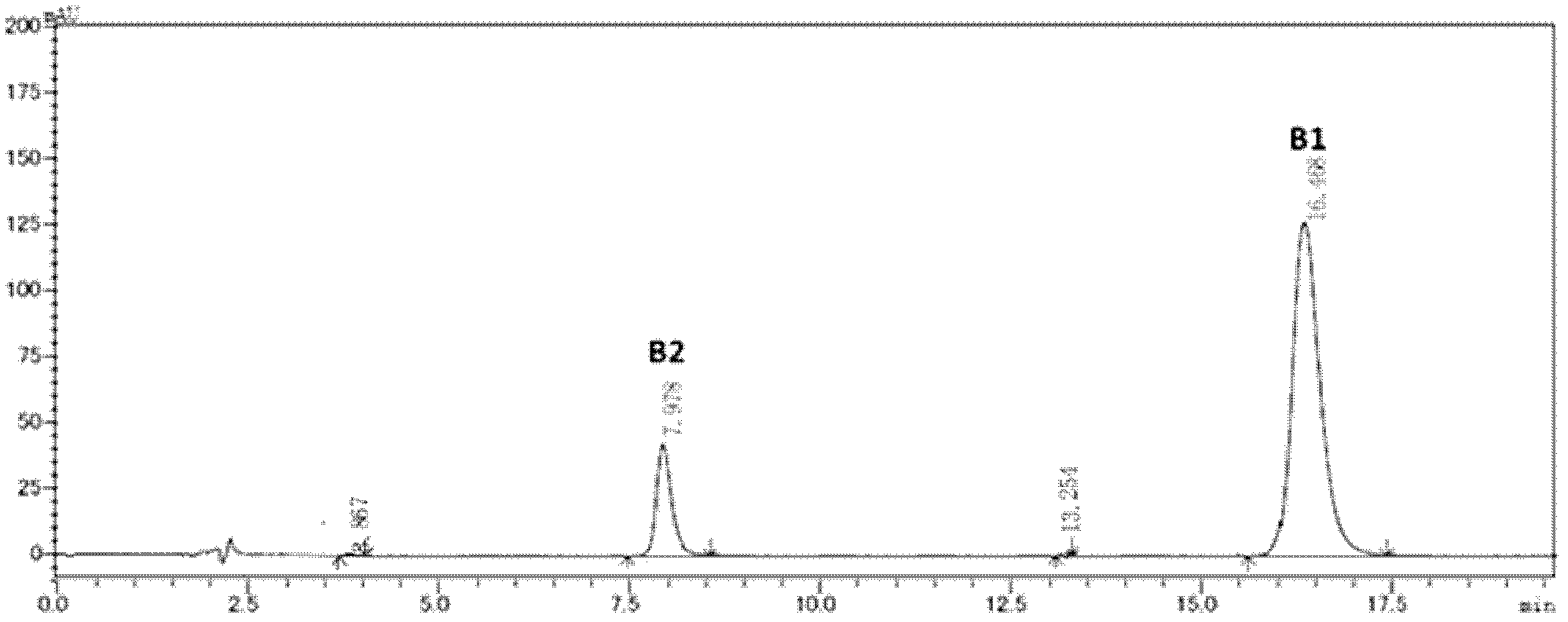
[0033] (2) Upper column separation: the chromatographic column is Kromasil 100-5-C8 (250×4.6mm), the particle diameter of packing C8 is 5 μm, the buffer solution is 35mM sodium sulfate aqueous solution (phosphoric acid adjusts pH=2.8), and the mobile phase is volume ratio Acetonitrile and buffer solution of 23:77, the clarified solution obtained in step (1) was injected, the flow rate was 1.2ml / min, the working temperature was 33°C, the pressure was 9.2MPa, the UV detection wavelength used was 215nm, and the target component B1 was collected .
[0034] The target component was concentrated by rotary evaporation at -0.1MPa and 50°C, acetonitrile and part of water were evaporated, and the concentrated solution was lyophilized, and...
Embodiment 3
[0036] Kromasil 100-3.5-C4 was used to replace Kromasil 100-5-C8 in Example 1, and the others were the same as in Example 1 to obtain polymyxin B2 with a purity of more than 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com