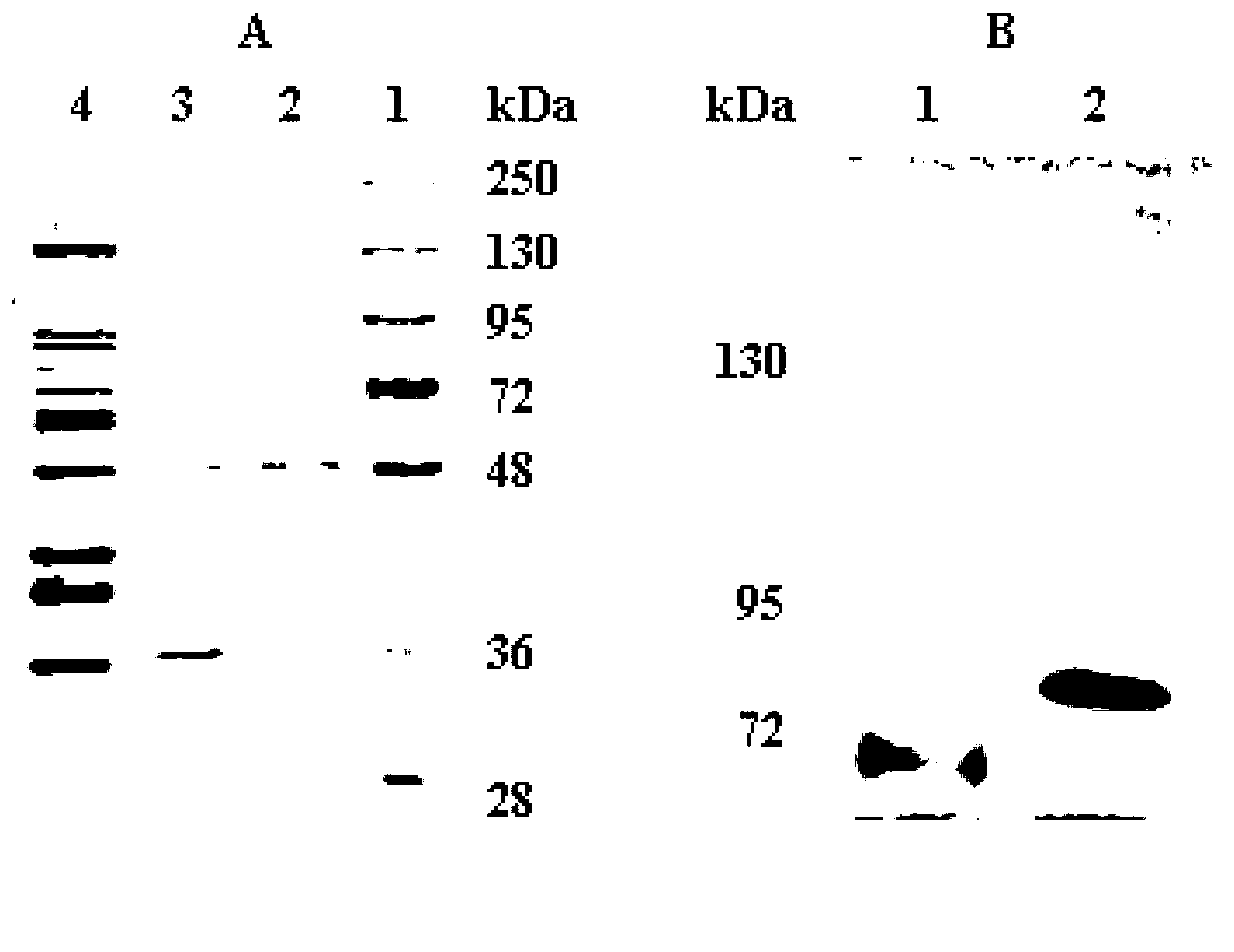
Marine-microbe-sourced low-temperature beta-galactosidase, and coding gene thereof and application thereof
A technology of galactosidase and marine microorganisms, applied in the direction of plant genetic improvement, application, genetic engineering, etc., can solve problems such as limited application, high optimum temperature, and rapid inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: A kind of preparation method of low-temperature β-galactosidase derived from marine microorganisms
[0053] 1. Fermentation of Halomonas sp.P6009-1
[0054] Inoculate the Halomonas sp.P6009-1 strain in Zobell2216E liquid medium (peptone 5g, yeast powder 1g, iron phosphate 0.1g, dissolved in 1L artificial seawater, pH7.4), 28°C, shaker (130rpm) for 24h to activate. The formula of artificial seawater is: NaCl25.0g, Na 2 SO 4 4.0g, KCl0.7g, NaHCO 3 0.20g, KBr0.10g, H 3 BO 3 0.03g, NaF0.003g, 53mL1.0mol / L MgCl 2 Solution, 10mL1.0mol / l CaCl 2 Solution, 0.90ml0.1mol / L SrCl 2 Solution, distilled water 1000ml. Then take 500 μL of the activated bacterial liquid and inoculate it into 100 mL of Zobell 2216E medium containing 2% lactose, and cultivate it at 28°C with shaking at 130 rpm for 4 days.
[0055] 2. Preparation of β-galactosidase crude enzyme solution of Halomonas sp.P6009-1
[0056] After the fermentation, the fermentation broth was centrifuged a...
Embodiment 2
[0059] Embodiment 2: The thermostability and optimal reaction temperature of the low-temperature β-galactosidase prepared in embodiment 1
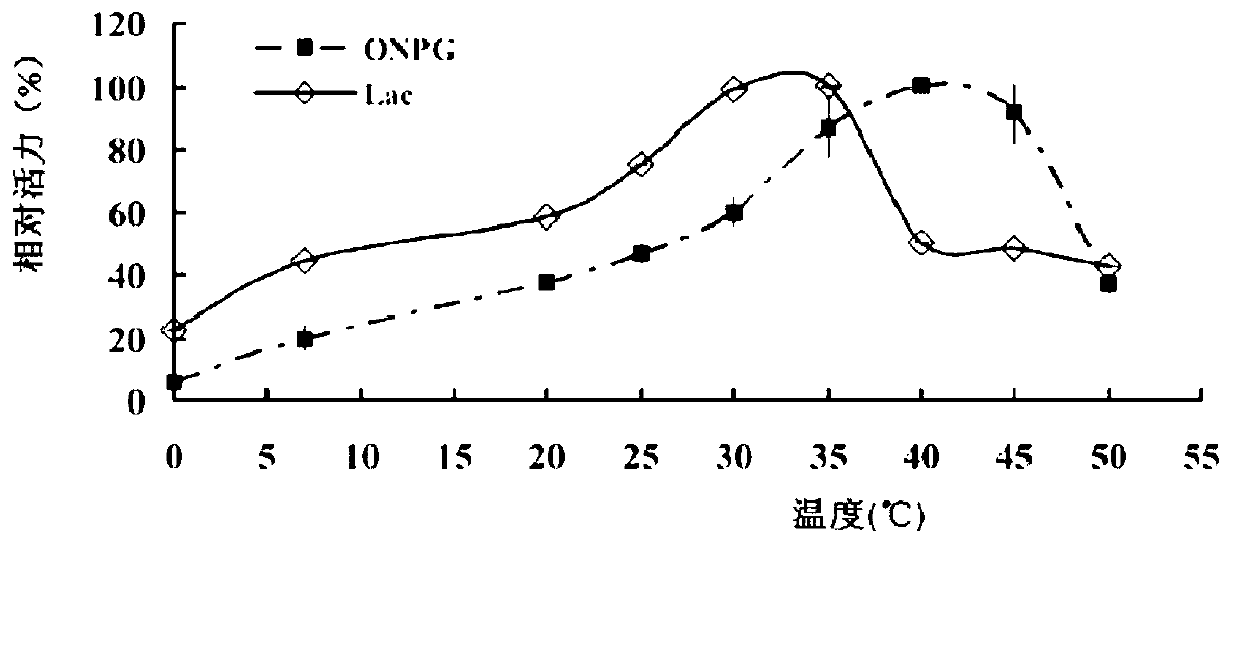
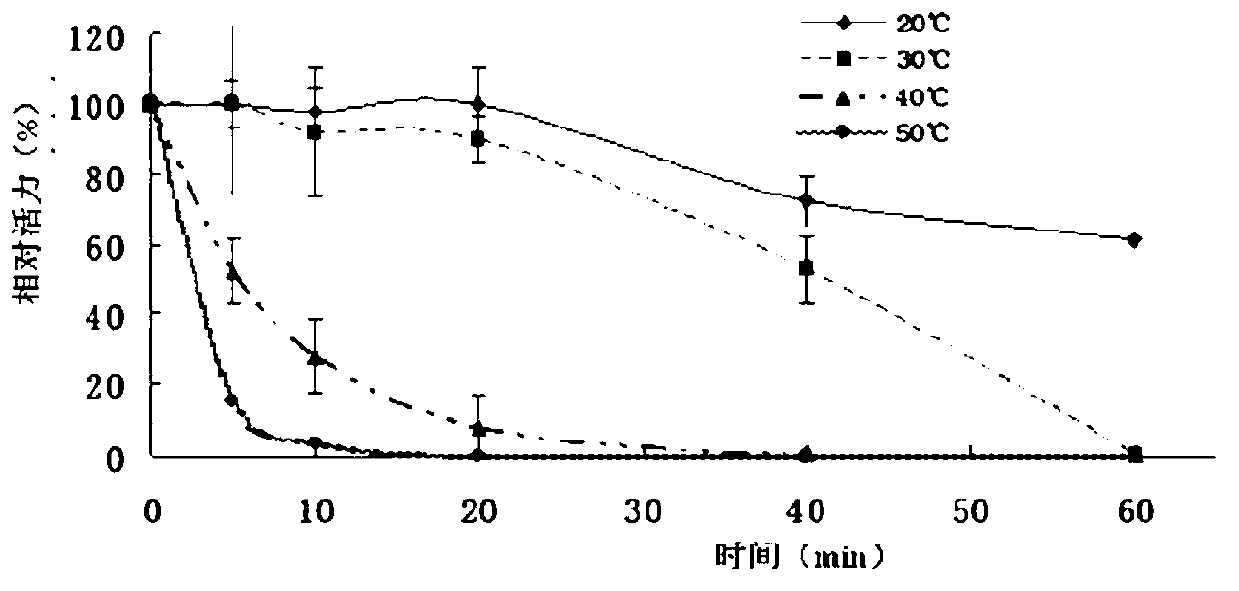
[0060] at 74 μl K 2 HPO 4 -KH 2 PO 4 (pH7.2, 0.1mol / L) buffer solution, add 1μL of LP6009-BGalH, 40mmol / LONPG or 25μl of lactose, place at 0-60℃ for 10min, then add 100μL of 1mol / L Na 2 CO 3 The reaction was terminated, and the absorbance value of ONP was measured at 410 nm or the amount of lactose was measured by HPLC. In thermostability experiments, the enzymes were pre-incubated at different temperatures of K 2 HPO 4 -KH 2 PO 4 (pH7.2, 0.1mol / L) buffer solution for 1 hour, and then carry out the above reaction. The ability to hydrolyze ONPG and lactose at the optimum temperature was determined. the result shows( figure 2 ), when ONPG was used as the substrate, the optimal reaction temperature of BGalH was 40°C; when lactose was used as the substrate, the optimal reaction temperature was 35°C. At 0°C, BGalH can maintain 5% a...
Embodiment 3
[0061] Embodiment 3: pH stability and optimum pH of the low-temperature β-galactosidase prepared in embodiment 1
[0062] Take 1 μL of P6009-BGalH, 74 μL of pH3.0-9.5 buffer (pH3.0-5.5 is 0.1mol / L citric acid-sodium citrate buffer; pH6.0-8.0 is 0.1mol / L K 2 HPO 4 -KH 2 PO 4 Buffer solution; pH8.5-9.5 is 0.1mol / L Tris-HCl buffer solution), 25μL of 40mmol / L ONPG dissolved in each of the above buffer solutions, mix well, keep the reaction solution containing BGalH at 30 ℃, and react 10min, then add 100μL of 1mol / L Na 2 CO 3 The reaction was terminated, and the absorbance value of ONP was measured at 410 nm or the amount of lactose was measured by HPLC. In the pH stability experiment, P6009-BGalH was pre-incubated in different pH buffers at 4°C for 1h, and then the above decomposition reaction was carried out at 30°C. The experimental results show( Figure 4 ), when ONPG was used as substrate, the optimum reaction pH of BGalH was 8.0; when lactose was used as substrate, the o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com