Method for synthesizing 2,4-dichlorphenoxyacetic acid
A technology of dichlorophenoxyacetic acid and chlorophenoxyacetic acid is applied in chemical instruments and methods, preparation of carboxylate, preparation of organic compounds, etc., to achieve the effects of low production cost, low odor, and simple processing flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
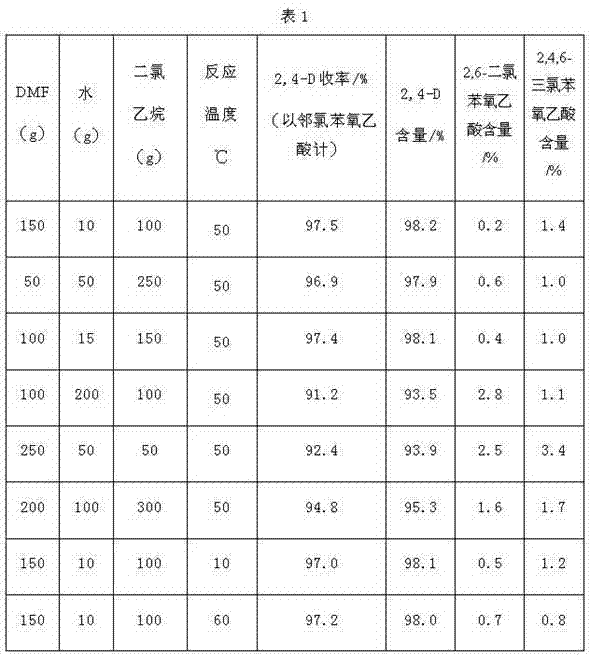
[0028] First, add 40g of water and 214g of 98% chloroacetic acid into a 500ml four-necked flask, and then dropwise add 205g of 32% liquid caustic soda, during which the temperature is controlled to be less than 60°C. Preparation of sodium chloroacetate solution. stand-by.
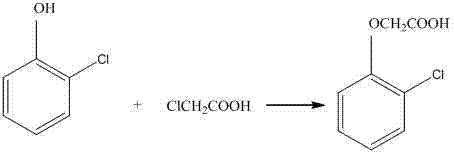
[0029] Add 262g of 98% o-chlorophenol into a 2000ml glass four-neck flask, then add 332.5g of 32% liquid caustic soda, then heat up to 60°C, then add the above prepared sodium chloroacetate solution, and then heat up to 95°C During the period, the pH is controlled at 9-11 by dropping liquid caustic soda, and the reaction is kept for 5 hours. During this period, 22g of 32% liquid caustic soda is added, and then the temperature is lowered to 80°C, and 267.8g of 30% hydrochloric acid is added dropwise, and then the temperature is lowered to 25°C, and filtered , dry to obtain 367.5g of o-chlorophenoxyacetic acid dry material, content 98.6%, the yield of o-chlorophenol is 97.1%.
[0030] Filtration obtained 93...
Embodiment 2
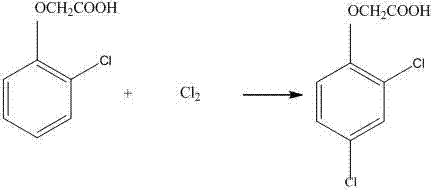
[0032] Take 100g of the dry material of o-chlorophenoxyacetic acid in Example 1, add it to a 1000ml four-necked flask, and then add 100g of DMF (N,N-dimethylformamide) to the flask, 15g of water, dichloro 150 g of ethane, and then mixed to dissolve the solid completely, then the temperature was raised to 30° C., and chlorine gas was introduced. After passing 38.5g of chlorine, take a sample test, the unconversion rate is 0.4%, then turn on the vacuum pump, control the vacuum degree in the flask to 0.09Mpa, then raise the temperature to remove the solvent, the end point temperature is 95°C, after removing 260g of solvent, add water to the flask 200g, began to cool down and crystallize. Cool down to room temperature, filter and dry to obtain 116.3g of 2,4-D dry material, 2,4-D content 97.1%, 2,6-dichlorophenoxyacetic acid content 0.5%, 2,4,6-trichlorobenzene The content of oxyacetic acid is 1.1%. This step yield is 96.8%. 212.6 g of filtrate was obtained. Add 20 g of dichlor...
Embodiment 3
[0034] Add 40g of water and 250g of 98% chloroacetic acid into a 500ml four-necked flask, and then dropwise add 205g of 32% liquid caustic soda, during which the temperature is controlled to be less than 60°C. Preparation of sodium chloroacetate solution. stand-by.
[0035] Add 257g of 98% o-chlorophenol into a 2000ml glass four-necked flask, and simultaneously add 15.8g of the alkali washing solution in Example 1, then add 319g of 32% liquid caustic soda, then heat up to 60°C, and then Add the above-prepared sodium chloroacetate solution, then raise the temperature to 110°C, during which the pH is controlled at 9-11 by dropwise adding liquid caustic soda, keep the temperature for 3 hours, add 19.9g of 32% liquid caustic soda during this period, and then cool down to 80°C, Add 268.2g of 30% hydrochloric acid dropwise, then cool down to 25°C, filter and dry to obtain 368.3g of dry o-chlorophenoxyacetic acid, with a content of 98.4%, and a yield of 97.23% based on o-chloropheno...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com