High performance liquid chromatography-mass spectrum-mass spectrum (HPLC-MS-MS) combined detection method for (S)-NNAL and (R)-NNAL in urine
A high-performance liquid chromatography and detection method technology, which is applied in the field of high-performance liquid chromatography-mass spectrometry-mass spectrometry detection, can solve problems such as difficult detection and complex urine matrix
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Unless otherwise stated, the percentages used in the present invention are all percentages by weight.
[0018] A high-performance liquid chromatography-mass spectrometry-mass spectrometry detection method for (S)-NNAL and (R)-NNAL in urine, the method comprising the following steps:
[0019] 1. Chemical reagents
[0020] (S)-(+)-α-methoxy-α-trifluoromethylphenylacetic anhydride (CAS No. 20445-33-4), (S)-(+)-α-methoxy-(three Fluoromethyl)phenylacetic anhydride (CAS No. 85541-57-7), 4-(-Methylnitrosoamino)-1-(3-pyridyl)-butanol (NNAL) (rac NNAL, CAS No. 76014- 81-8), 4-(methyl-d3-nitrosoamino)-1-(3-pyridyl)-1-butanol (rac-NNAL-methyl-d3CAS No. 1020719-61-2), triethyl Amylamine, β-glucosidase (type IX-A, from Escherichia coli).
[0021] Preparation of (S)-NNAL and (R)-NNAL and determination of absolute configuration: preparative OD-H chiral chromatography column, mobile phase isopropanol: n-hexane ratio of 10:90, two effluents were collected Chromatographic peaks were ...
Embodiment 2
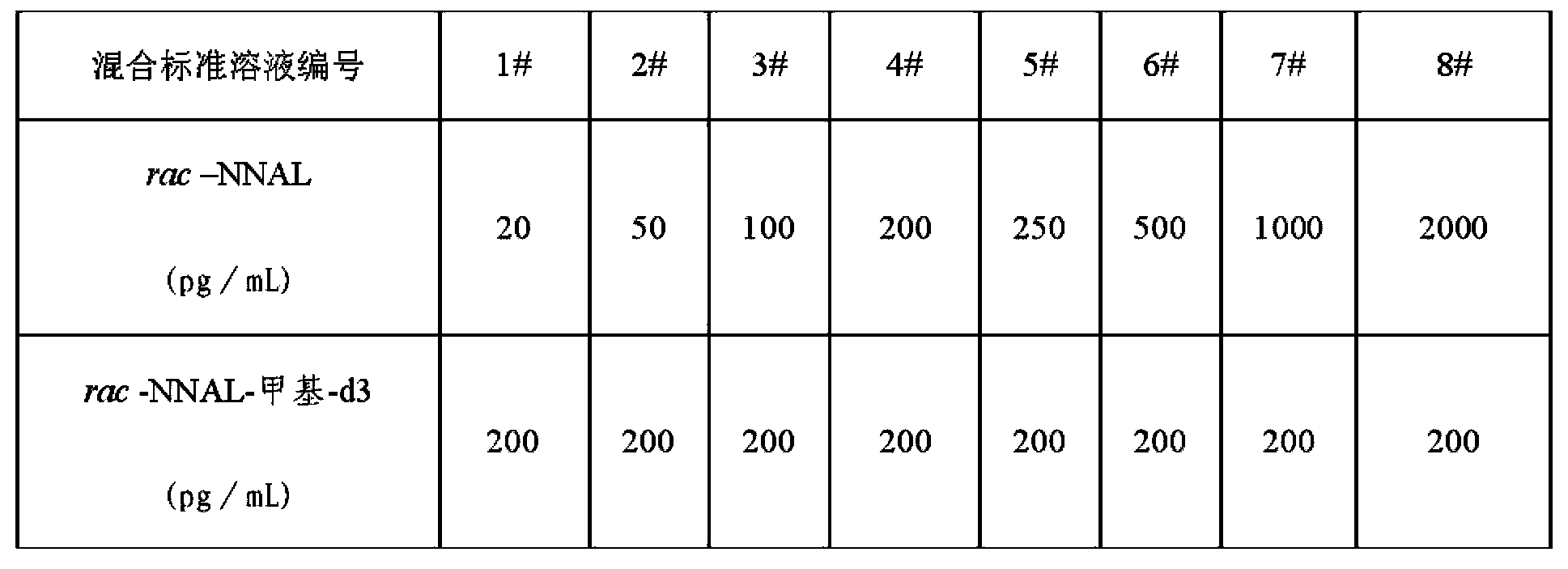
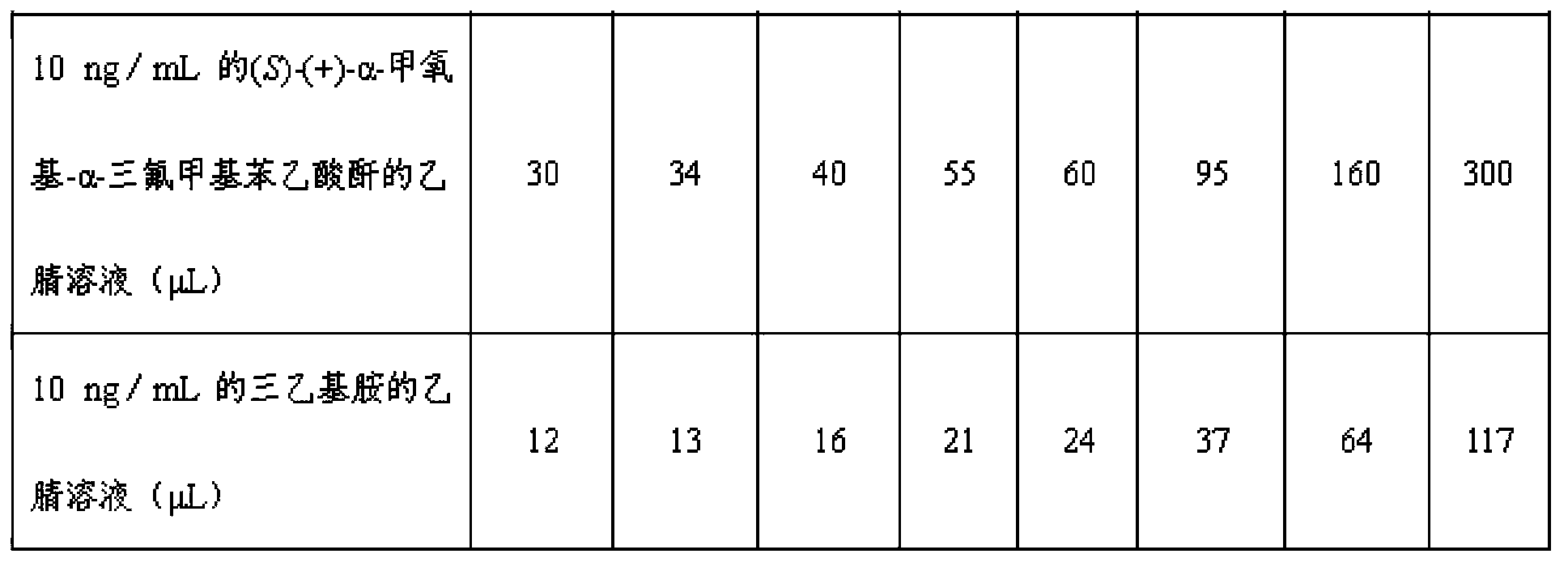
[0039] 1. Derivatization of racemic NNAL: use anhydrous acetonitrile as a solvent to prepare a single standard solution of rac NNAL standard with a concentration of 1 μg / mL, and keep four significant figures; prepare in the same way using anhydrous acetonitrile as a solvent A single standard solution of rac-NNAL-methyl-d3 with a concentration of 1 μg / mL; using anhydrous acetonitrile as a solvent, prepare a mixed standard solution containing rac-NNAL and deuterated rac-NNAL-methyl-d3 according to Table 1; Prepare anhydrous acetonitrile solution of (S)-(+)-α-methoxy-α-trifluoromethylphenylacetic anhydride with a concentration of 10ng / mL; prepare anhydrous solution of triethylamine with a concentration of 10ng / mL Acetonitrile solution; respectively take 0.5mL mixed standard solution in HPLC-MS-MS chromatographic bottles, and then add (S)-(+)-α-methoxy-α-trifluoromethylphenylacetic anhydride according to Table 2 Anhydrous acetonitrile solution and triethylamine acetonitrile soluti...
Embodiment 3
[0043] 1. Preparation of (S)-NNAL and (R)-NNAL and determination of absolute configuration: use anhydrous acetonitrile solution of rac NNAL standard, (S)-(+)-α-methoxy-α-tri The anhydrous acetonitrile solution of fluoromethylphenylacetic anhydride and the anhydrous acetonitrile solution of (R)-(+)-α-methoxy-α-trifluoromethylphenylacetic anhydride and triethylamine carry out Mosher reaction respectively, and then After purification and 1H NMR spectrum test, Mosher method was used to determine (S)-NNAL-(S)-(+)-α-methoxy-α-trifluoromethylphenylacetate and (R)-NNAL- Absolute configuration of (S)-(+)-α-methoxy-α-trifluoromethylphenylacetate.
[0044] 2. Derivatization of racemic NNAL and establishment of a standard curve: use anhydrous acetonitrile as a solvent to prepare a single standard solution of rac NNAL standard with a concentration of 1 μg / mL, and keep four significant figures; Prepare a single standard solution of rac-NNAL-methyl-d3 with a concentration of 1 μg / mL in acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com