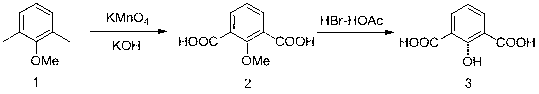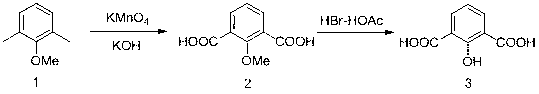Preparation method of 2-hydroxyisophthalic acid
A technology of hydroxyisophthalic acid and methoxyisophthalic acid, which is applied in the field of preparation of 2-hydroxyisophthalic acid, can solve problems such as increased reaction energy consumption, high requirements for reactors, and large risk factor, and achieves The effect of short reaction time, low equipment requirements and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of preparation method of 2-hydroxyisophthalic acid, adopts following synthetic route to make:
[0024]
[0025] The specific steps are:
[0026] (1) Add 3.3 g (0.06 mol) of potassium hydroxide, 98 ml (5.4 mol) of water and 19.15 g (0.121 mol) of potassium permanganate to the reactor in sequence, stir and dissolve to obtain an alkaline aqueous solution of potassium permanganate. And slowly add 2.6 ml (0.018 mol) of 2,6-dimethylanisole dropwise to the alkaline aqueous solution of potassium permanganate under stirring to make it fully dispersed.
[0027] (2) Heat up the temperature to 80 oC and reflux under stirring, and the reaction time is 4 h; after the reaction is completed, cool the reaction solution to room temperature, filter it with suction, and then add concentrated hydrochloric acid to the filtrate under stirring to acidify the system until the pH of the system is 3- 4. A large amount of white solid was precipitated, which is the intermediate 2-methoxy...
Embodiment 2
[0034] Carry out according to the method step of embodiment 1, its synthetic route is:
[0035]
[0036] The specific steps are:
[0037] (1) Add 3.3 g (0.06 mol) of potassium hydroxide, 83 ml (4.5 mol) of water and 20.0 g (0.126 mol) of potassium permanganate to the reactor in sequence, stir and dissolve to obtain an alkaline aqueous solution of potassium permanganate. And slowly add 2.6 ml (0.018 mol) of 2,6-dimethylanisole dropwise to the alkaline aqueous solution of potassium permanganate under stirring to make it fully dispersed.
[0038] (2) Heat up the temperature to 80 oC and reflux under stirring, and the reaction time is 4 h; after the reaction is completed, cool the reaction solution to room temperature, filter it with suction, and then add concentrated hydrochloric acid to the filtrate under stirring to acidify the system until the pH of the system is 3- 4. A large amount of white solid was precipitated, which is the intermediate 2-methoxyisophthalic acid, filt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com