Hydroxamic acid compound, and preparation method and application thereof
A compound, hydroxamic technology, applied in the field of hydroxamic acid, can solve the problems of toxic reaction, HDACs subtype selectivity, poor bioavailability, etc., to achieve mild reaction conditions, affect cell cycle arrest, and few steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Preparation of N-(2,5-dimethoxyphenyl)-N′-hydroxysuberamide (abbreviation: N25)
[0061] The reaction scheme is as follows:
[0062]
[0063] S1. Weigh 3.158g (20mmol) of 2,5-dimethoxyaniline and 9.031g (20mmol) of Carter’s condensing agent BOP into a 250ml round bottom flask, add 130ml of anhydrous dichloromethane, stir and mix, and then Add 3.670ml (20mmol) of monomethyl suberate and 12.440ml (70.67mmol) of DIEA, mix well, put it at room temperature, and stir it with magnetic force. The reaction time is 24h, and TLC detection is carried out during the period. The developer is ethyl acetate : Petroleum ether=1:2. Remove the solvent by rotary evaporation, use 100-200 mesh silica gel column for chromatography, and carry out separation and purification on the silica gel column. Reobtain 2.124g brown oil, productive rate: 32.88%;
[0064] S2. Weigh NH 2 OH·HCl 3.712g was dissolved in 20mL methanol, NaOH 5.261g was dissolved in methanol, and the two were mi...
Embodiment 2
[0067] Example 2 Preparation of N-(2-phenoxyphenyl)-N'-hydroxysuberamide
[0068] The reaction scheme is as follows:
[0069]
[0070] S1. Weigh 0.561g (3mmol) of 2-phenoxyaniline and 0.576g (3mmol) of Carter’s condensing agent BOP into a 50ml round bottom flask, add 20ml of anhydrous dichloromethane, stir and mix well, then add octane Acid monomethyl ester 0.550ml (3mmol), DIEA 1.865ml (10.6mmol), after mixing, put it at room temperature, stir with a magnetic force, the reaction time is 24h, TLC detection is carried out during this period, the developer is ethyl acetate: petroleum ether =1:2. The solvent was removed by rotary evaporation, and the silica gel column used for chromatography of 100-200 mesh was used for separation and purification of the silica gel column. The eluent was ethyl acetate:petroleum ether=1:20, and a yellow-brown oily solution was obtained. Weighed to obtain 0.689g, yield: 64.69%.
[0071] S2. Weigh NH 2 1.095 g of OH·HCl was dissolved in 20 mL...
Embodiment 3
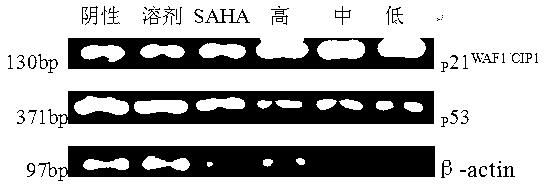
[0073] Example 3 Anti-proliferation effect of N25 on tumor cells
[0074] Three glioma cell lines: U251, U87, T98G and three other lung cancer cell lines: H460, A549, H1299 were selected, and the anti-proliferation experiments were conducted on the cells by CCK-8 method. Cells in good growth state were inoculated in 96-well plates at a cell density of 10 4 Each well, N25 and the control drug SAHA were pre-acted on the same cell, and each concentration of each drug was set to 3 replicate holes. After the cells adhered to the wall, the drug treatment was performed. : 0.5umol / L, 1.0umol / L, 2.0umol / L, 4.0umol / L, 8.0umol / L, 16.0umol / L, 32.0umol / L, after administration for 48 hours, add 10ul CCK-8 solution to each well , shake well, put into the cell incubator and incubate for 1 h, and measure the absorbance value at 450nm on a microplate reader. The results are shown in Table 1 and Table 2.
[0075] The results showed that the sensitivity of N25 to different tumor cell lines was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com