Method for preparing nitazoxanide
A technology of nitazoxanide and nitrothiazole, which is applied in the field of preparation of nitazoxanide, can solve the problems of difficult handling, high production cost, and high cost, and achieve the effects of easy control, reduced production cost, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
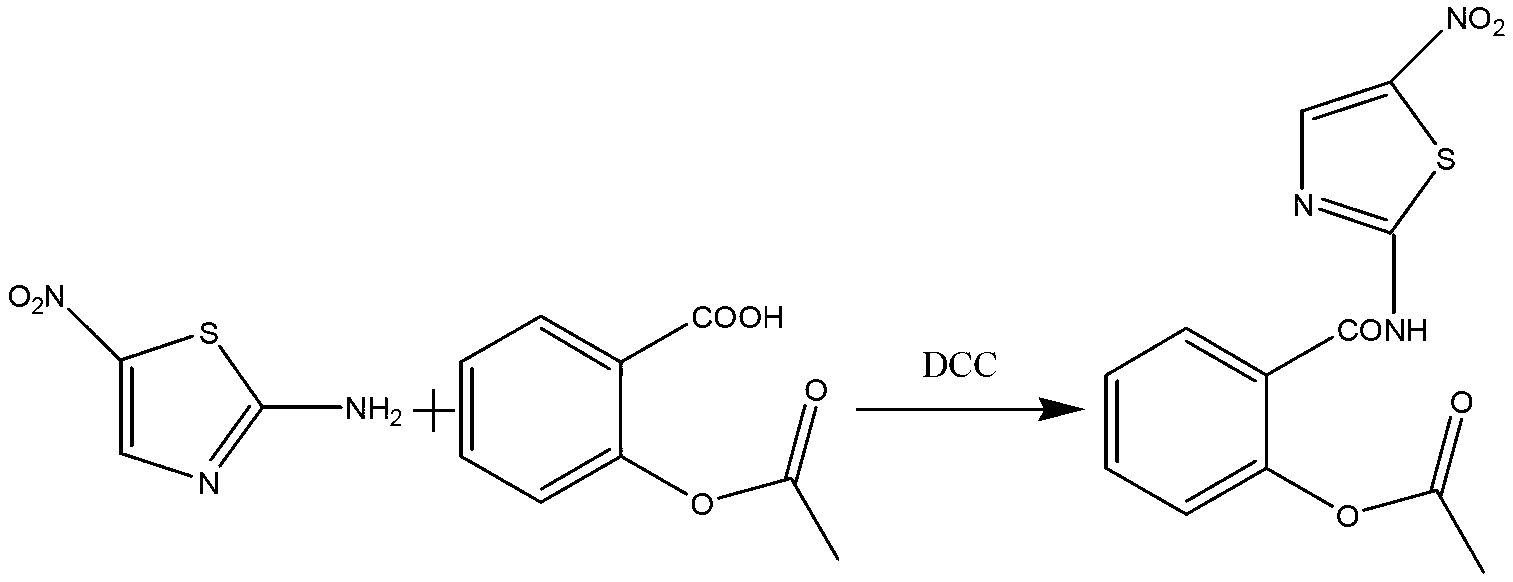
[0028] The preparation process of nitazoxanide comprises the following two steps:
[0029] (1) Synthesis of o-acetylsalicyloyl chloride:
[0030] In a 50L reaction kettle, add 8KG of acetylsalicylic acid and 6.34KG of thionyl chloride, mechanically stir, control the reaction temperature between 50°C and 60°C, and stir for 2 hours to obtain o-acetylsalicylic acid chloride. Concentrate under reduced pressure to remove excess thionyl chloride, and then add anhydrous 1,4-dioxane 16KG to prepare a solution of o-acetylsalicyloyl chloride for future use.
[0031] (2) Synthesis of nitazoxanide:
[0032] In a 100L reaction kettle, add 45L of anhydrous 1,4-dioxane, 5.37KG of 2-amino-5-nitrothiazole, and 3.74KG of triethylamine, cool down to -2°C, and control the temperature at -2°C~ Add the o-acetylsalicyloyl chloride solution obtained in step (1) dropwise at 2°C, raise the temperature to 20°C-25°C after the dropwise addition, stir for 30 minutes, then filter, and wash the filtrate wi...
Embodiment 2
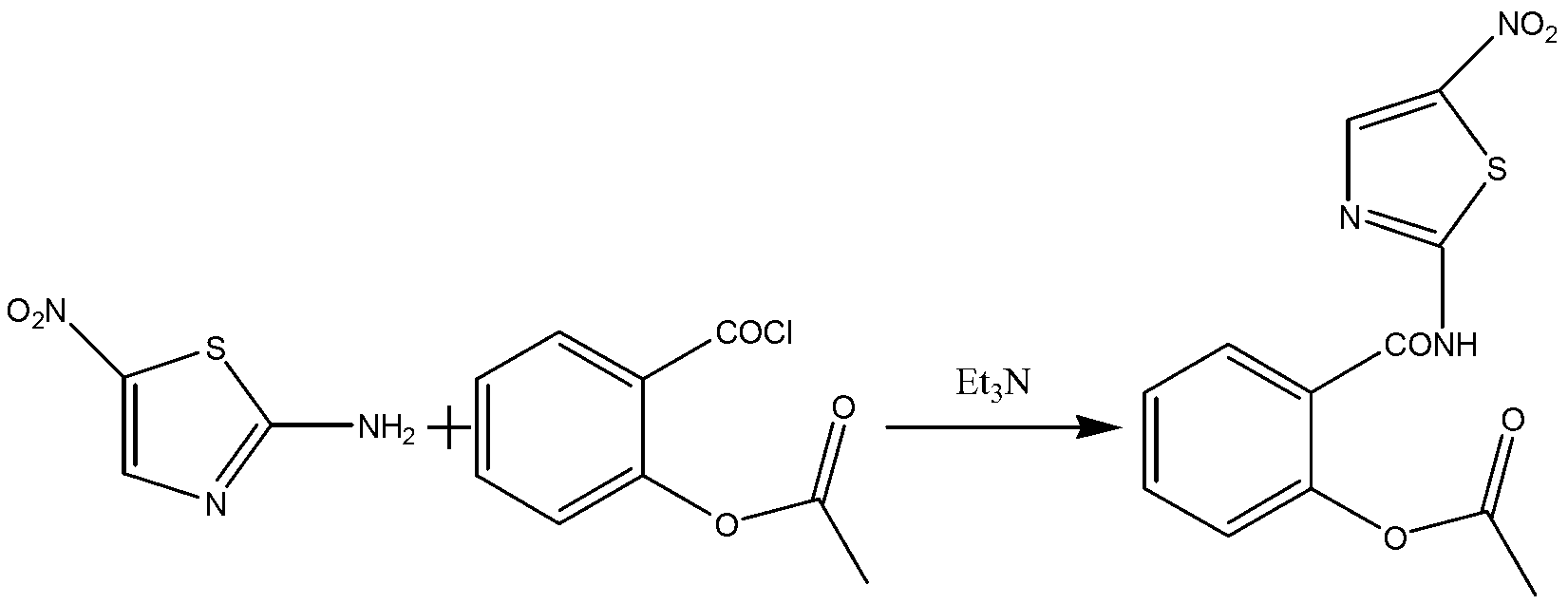
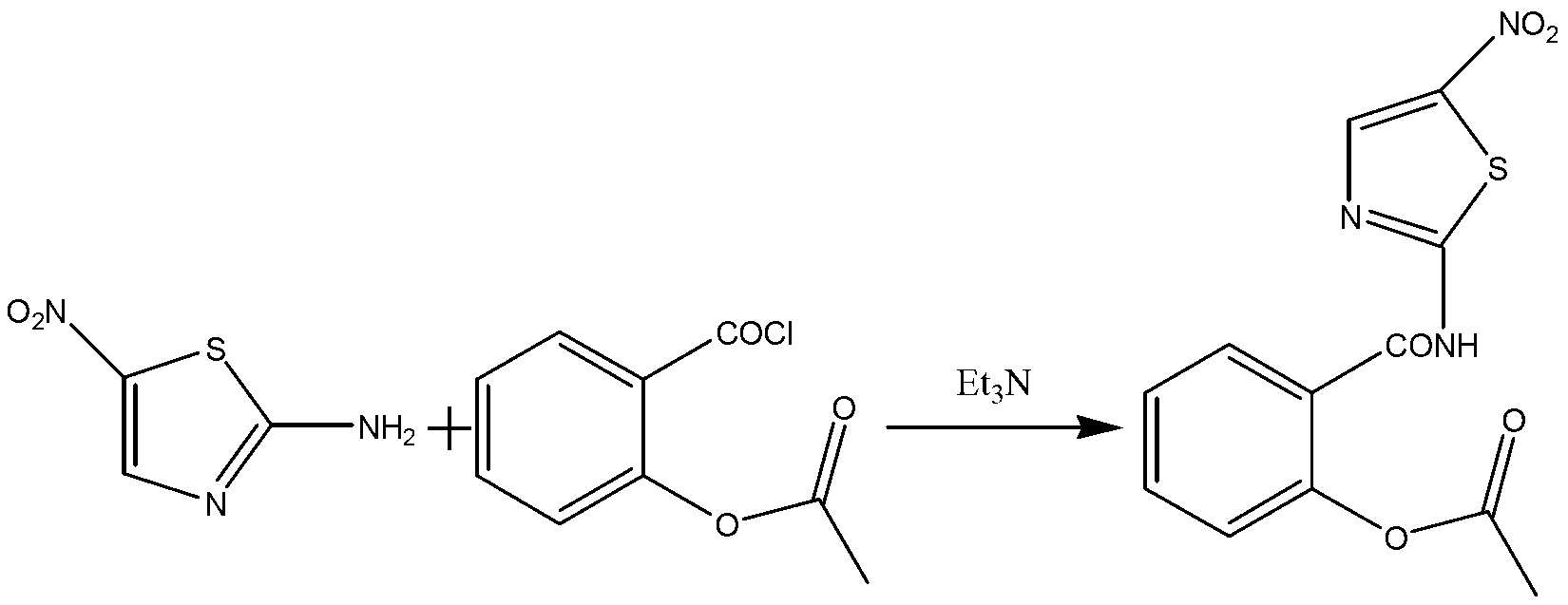
[0034] The preparation process of nitazoxanide comprises the following two steps:
[0035] (1) Synthesis of o-acetylsalicyloyl chloride:
[0036] In a 100L reaction kettle, add 16KG of acetylsalicylic acid and 10.6KG of thionyl chloride, stir mechanically, control the reaction temperature at 65°C to 70°C, and stir for 2.5h to obtain o-acetylsalicylic acid chloride. Concentrate the excess chlorine sulfoxide, and then add N,N-dimethylformamide (DMF) 32KG to prepare a solution of o-acetylsalicyloyl chloride for later use.
[0037] (2) Synthesis of nitazoxanide:
[0038] In a 200L reactor, add 46L of N,N-dimethylformamide (DMF), 9.02KG of 2-amino-5-nitrothiazole and 6.29KG of triethylamine, cool down to -2°C, and control the temperature at -2 ℃~2℃, add the o-acetylsalicyloyl chloride solution obtained in step (1) dropwise, raise the temperature to 20℃~25℃ after the dropwise addition, stir for 60min and then filter, and wash the filtrate with 40KG water to obtain the crude produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com