Oxyresveratrol synthesis method
A technology for oxidizing asparagine and a synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of long synthesis route and only 30% total yield, and achieves high yield and raw material. Easy-to-obtain, maneuverable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
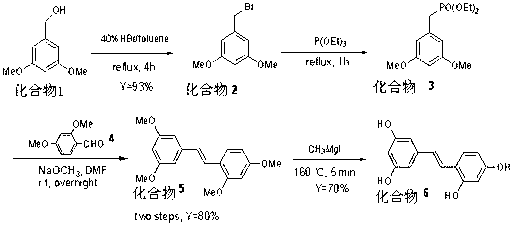
[0022] The reaction equation is:
[0023]
[0024] The present invention is a kind of synthetic method of oxystilbenzyl bromide, comprising 3,5-dimethoxybenzyl bromide (compound 2 ), 2,3',4,5'-tetramethoxystilbene (compound 5 ) synthesis, oxystilbene triphenol (compound 6 )Synthesis.
[0025] 3,5-dimethoxybenzyl bromide (compound 2 )Synthesis
[0026] Add 40% HBr (24 mL, 0.12 mol) to a solution of 3,5-dimethoxybenzyl alcohol (16.8 g, 0.1 mol) in toluene (50 mL), and react at 80°C for about 4 h. After the completion of the reaction was confirmed by TLC, the toluene was spun off, and the remaining turbid solution was extracted three times with ethyl acetate (60 mL), the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was spun off to obtain a reddish-brown oily liquid. White crystals of 21.3 were obtained after recrystallization with ethyl acetate and petroleum ether, yield = 93.0%. 1 H NMR (CDCl 3 ): 6.54 (s, 2H), 6.39 (s, 1H), 4.42 (...
Embodiment 2
[0032] 3,5-dimethoxybenzyl bromide (compound 2 ), (Z)-2,3',4,5'-tetramethoxystilbene (compound 5 ) Synthesis, as described in Example 1.
[0033] Oxystilbene triphenols (compound 6 )Synthesis
[0034] In a 250 mL round-bottomed flask, add magnetite, magnesium chips (5.5 g, 0.23 mol) and anhydrous ether (30 mL), and continuously add a solution of methyl iodide (123.5 g, 0.87 mol) in ether (20 mL) under stirring. ) until the magnesium chips are completely dissolved. Then add to the reaction bottle 5 (3, 5, 2’, 4’)-tetramethoxystilbene (3 g, 0.01 mol), stirred at room temperature until completely dissolved, and the solvent was removed by spin to obtain a dark gray viscous liquid. Then react at 160 °C for 5 min, cool slowly, crush the obtained reaction system, add anhydrous ethyl acetate (50 mL) to it, and then add ice water (50 mL), and wash the water layer with ethyl acetate ( 20 mL) was extracted three times, the organic phases were combined, dried over sodium sulfate, an...
Embodiment 3
[0036] 3,5-dimethoxybenzyl bromide (compound 2 ), (Z)-2,3',4,5'-tetramethoxystilbene (compound 5 ) Synthesis, as described in Example 1.
[0037] Oxystilbene triphenols (compound 6 )Synthesis
[0038] In a 250 mL round-bottomed flask, add magnetite, magnesium chips (1.92 g, 0.08 mol) and anhydrous ether (20 mL), and continuously add a solution of methyl iodide (12.8 g, 0.09 mol) in ether (20 mL) under stirring. ) until the magnesium chips are completely dissolved. Then add the compound to the reaction vial 5 (3, 5, 2’, 4’)-tetramethoxystilbene (3 g, 0.01 mol), stirred at room temperature until completely dissolved, and the solvent was removed by spin to obtain a dark gray viscous liquid. Then react at 160 °C for 5 min, cool slowly, crush the obtained reaction system, add anhydrous ethyl acetate (50 mL) to it, and then add ice water (50 mL), and wash the water layer with ethyl acetate ( 20 mL) was extracted three times, the organic phases were combined, dried over sodium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com