Application of furocoumarin compounds in preparation of anti-hepatitis B virus (HBV) medicaments
A technology of hepatitis B virus and furanocoumarin, which is applied in the direction of antiviral agents, active ingredients of heterocyclic compounds, etc., can solve the problems of lack of drug resistance and achieve the effect of simple structure, low toxicity and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: computer drug screening
[0046] Compound library preprocessing: The compound library is a self-prepared compound database. The compound library is processed as follows: removing ions and complexing water molecules, adding charges, protonating, and generating three-dimensional conformations. These processes were completed in the drug design software package Discovery Studio 2.5. The protonation is carried out at pH 6.5~8.5. Prepare small molecule libraries for virtual screening.
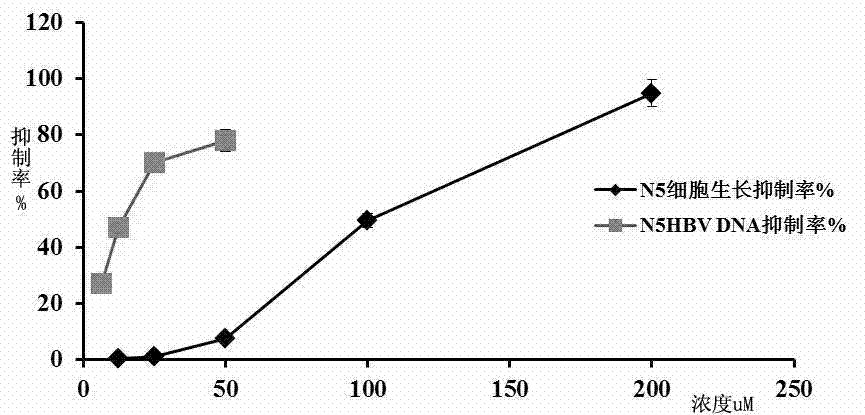
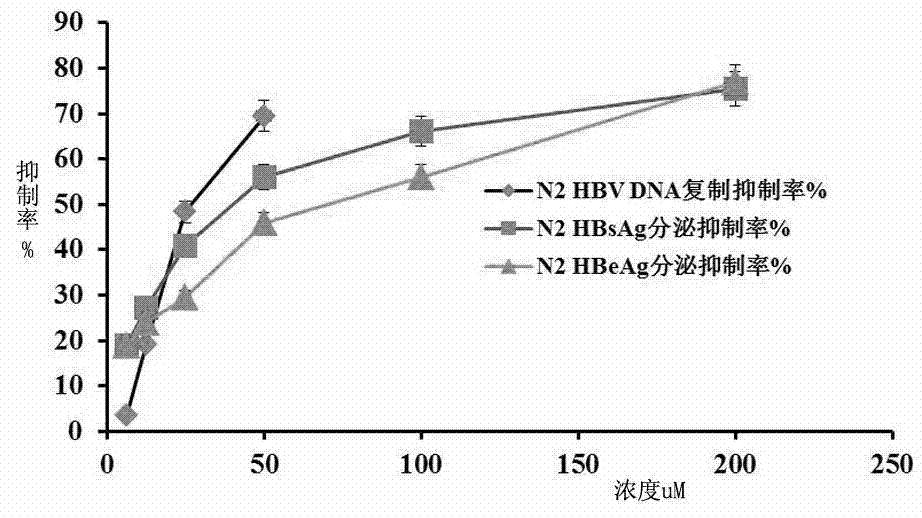
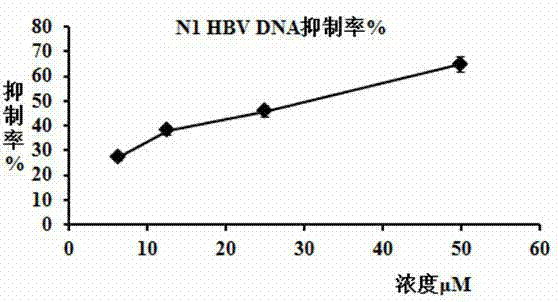
[0047] A substructure search was performed with the furanocoumarin structure to find compounds containing the parent nucleus. The present invention predicts that there are 13 candidate inhibitors of human NF-κB target in the compound reserve library of the laboratory through the computer drug screening program DiscoveryStudio version 2.5. The 13 compounds were screened for their anti-HBV replication activity at the cellular level, and 8 of them (N1-N8) were found to inhibit H...
Embodiment 2
[0052] Example 2: Determination of the Toxicity of Compounds to Host Cells
[0053] S1. Cell culture.
[0054] HepG2.2.15 cells (human liver cancer cells transfected with HBV gene, which can stably express HBV virus particles and HBV-related proteins) were cultured in vitro. Use RPMI 1640 medium containing 10% fetal bovine serum and 200mg / L G418 at 37°C and 5% carbon dioxide concentration for routine maintenance and passage.
[0055] S2. Compound intervention.
[0056] Collect logarithmic phase cells and make the cell suspension concentration 5×10 4 cells / ml, added to 96-well cell culture plate. After culturing in a carbon dioxide incubator for 24 hours, the culture solution was replaced with a medium containing different compound concentrations, and the culture was continued for 6 days, and the medium with the same compound concentration was replaced every 3 days. The cytotoxicity was detected on the 6th day, and DMSO was used to The compounds to be tested are formulated ...
Embodiment 3
[0064] Example 3 N1 ~ N8 compounds in Table 1 inhibit the expression of HBsAg and HBeAg antigens
[0065] S1. Cell culture.
[0066] HepG2.2.15 cells were cultured in vitro. Use RPMI 1640 medium containing 10% fetal bovine serum and 200mg / L G418 at 37°C and 5% carbon dioxide concentration for routine maintenance and passage.
[0067] S2. Drug intervention.
[0068] Adjust the logarithmic growth phase HepG2.2.15 cell density to 5×10 4 cells / ml, seeded in a 96-well culture plate, 100 μl per well, after 24 hours, the cells were attached to the bottom of the well, the supernatant was discarded, and the culture solution containing different concentrations of drugs was added, and 3 replicate wells were set for each concentration. Add the cells with complete medium as normal control, 5% CO 2 , Cultivate at 37°C, and change the concentration of the same drug solution once every three days.
[0069] S3. Test method.
[0070] The cell supernatant was collected on the 6th day, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com