Preparation method of side chain type imidazolyl benzdine
A technology of imidazole-based benzidine and hydroxybenzidine, which is applied in the field of preparation of aromatic diamine monomers, can solve the problem of poor hydrolytic stability of sulfonated polyimide, few varieties of imidazole-based diamine monomers, and restrictions on imidazole-based Polymer development and other issues to achieve the effect of improving polymer performance and enriching types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
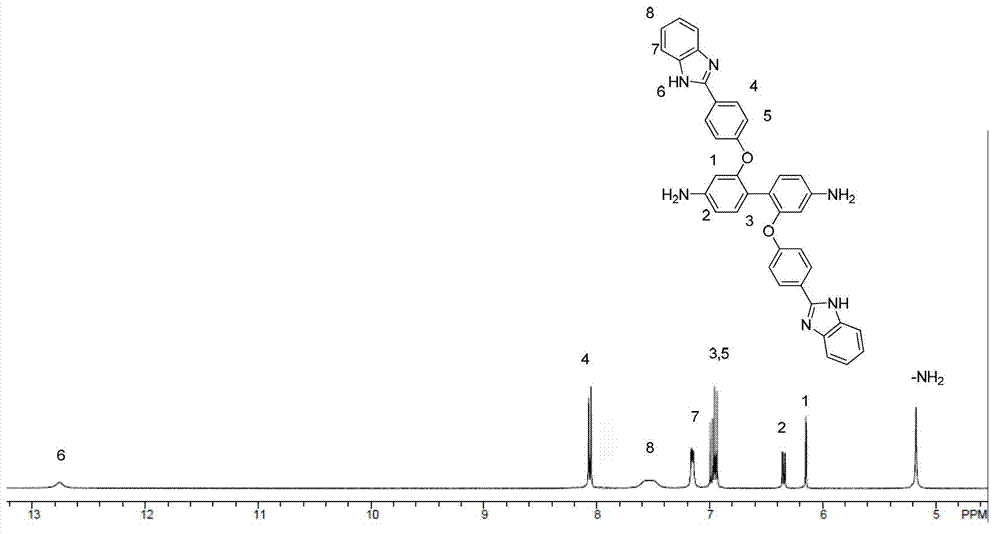
[0032] Synthesis of 2,2'-bis(4-(1H-benzimidazol-2-yl)phenoxy)benzidine
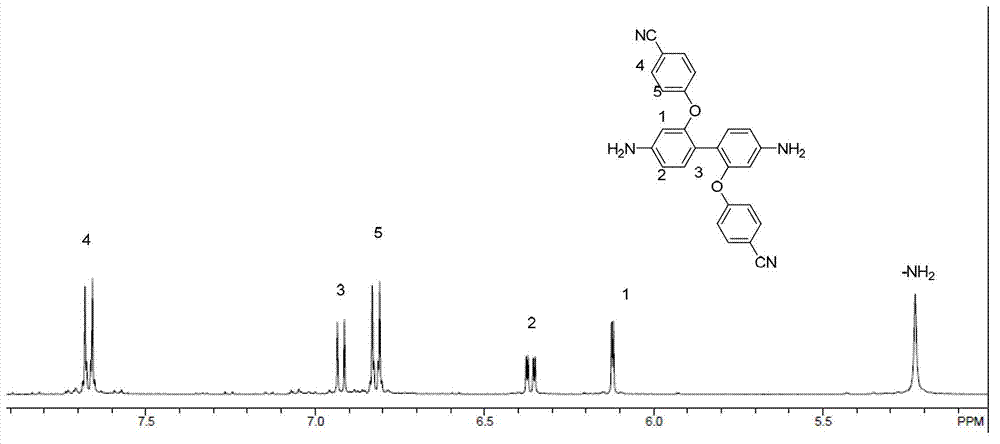
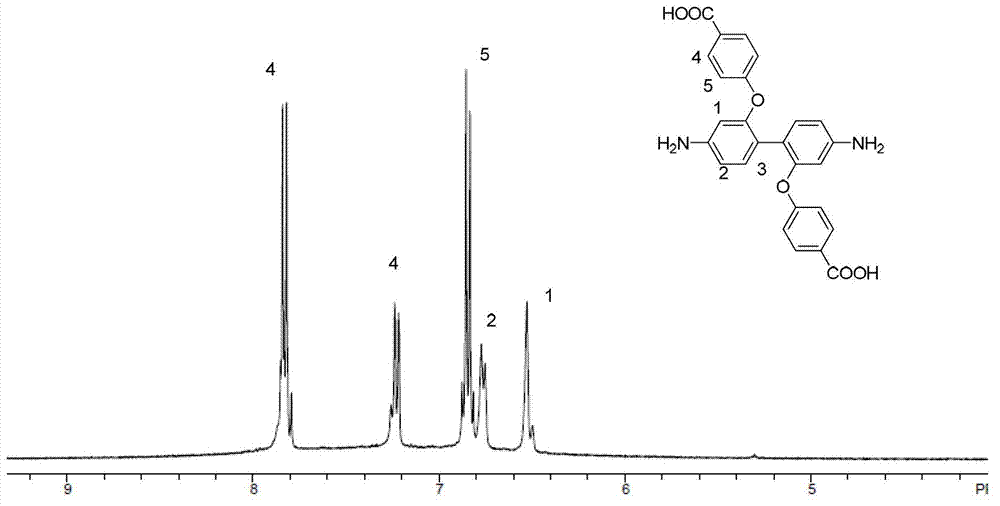
[0033] (1) Under nitrogen protection, add 2.16g (0.010mol) of 2,2'-dihydroxybenzidine and 2.42g (0.020mol) of p-fluorobenzidine into a 100mL three-necked flask equipped with a water separator and fully dried Cyanide, 3.50g (0.025mol) potassium carbonate, 15mL N,N-dimethylacetamide, stir evenly and heat to 140°C, slowly add 10mL toluene dropwise with a constant pressure dropping funnel, react at this temperature for 4 hours and The toluene and water were distilled off completely. Then the temperature was raised to 150° C. for 20 hours of reaction. After the reaction, the system was cooled to room temperature and poured into deionized water. A large amount of reddish-brown solids were precipitated. Filtered with suction to collect the filter cake. The filter cake was washed repeatedly with deionized water and vacuum-dried at 60°C for 12 hours to obtain a reddish-brown solid. , and the yield was 85%.
[0...
Embodiment 2
[0040] Preparation of Side Chain Type Imidazolyl Sulfonated Polyimide Proton Exchange Membrane
[0041] (1) Under nitrogen protection, add 0.9504g (1.8mmol) of 4,4'-bis(4-aminophenoxy)biphenyl-3,3'-disulfonic acid ( BAPBDS), 0.1200g (0.2mmol) 2,2'-bis(4-(1H-benzimidazol-2-yl)phenoxy)benzidine, add 0.7mL triethylamine, 15mL m-cresol, stir to make The solid dissolves. After the solid is completely dissolved, add 0.5360g (2mmol) of 1,4,5,8-naphthalene tetracarboxylic dianhydride (NTDA), 0.4884g of benzoic acid, react at 80°C for 8 hours, and then heat up to 180°C React for 20 hours. The system was cooled to room temperature, poured into 150 mL of methanol to obtain a reddish-brown fibrous solid, which was filtered with suction, and the solid was dried in vacuum at 60°C.
[0042] (2) Add 0.5 g of the polymer synthesized in step (1) to a 25 mL Erlenmeyer flask, and add 10 mL of m-cresol to dissolve it completely. Filter, cast the filtrate on a glass plate, and dry it at 110°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com