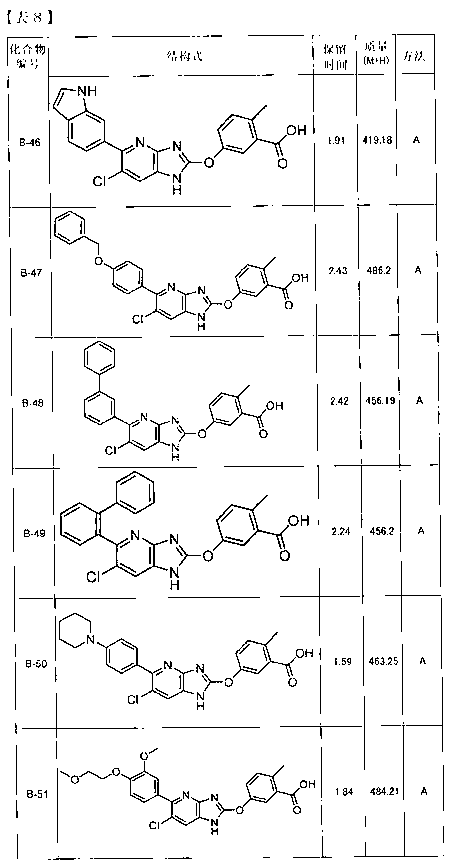
Hetero ring-fused imidazole derivative having ampk activating effect
A compound and heterocyclic group technology, applied in the field of heterocyclic fused imidazole derivatives with AMPK activation, can solve the problem that AMPK activation is not documented
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1160] 【chemical 70】
[1161]
[1162] While stirring a suspension of 6-chloro-3-nitropyridin-2-amine 1 (20 g, 115 mmol) in absolute ethanol (970 mL), chlorine gas was bubbled at 0°C for 1 hour. Then, stirring the reaction liquid, after bubbling nitrogen gas at room temperature for 1 hour, it stirred at 0 degreeC for 30 minutes. The reaction suspension was collected by filtration and washed with diisopropyl ether, whereby a solid was obtained. The solvent of the obtained filtrate was distilled off under reduced pressure, and the precipitated solid was collected by filtration and washed with diisopropyl ether to obtain a further solid. Compound 2 (18.1 g, 76%) was obtained as a yellow solid by combining the above two solids collected by filtration.
[1163] Compound 2; 1 H-NMR (DMSO-d 6 ) δ: 8.33 (brs, 2H), 8.59 (s, 1H).
[1164] Add iron (48.6 g, 870 mmol) and ammonium chloride (46.5 g, 870 mmol) to compound 2 (36.2 g, 174 mmol) in ethanol (775 mL), water (310 mL) and st...
Embodiment 2
[1180] 【Chemical 71】
[1181]
[1182] Compound A-2; 1 H-NMR (DMSO-d 6 ) δ: 2.56 (s, 3H), 3.84 (s, 3H), 6.51 (d, J = 4.0 Hz, 1H), 7.38-7.45 (m, 3H), 7.49-7.51 (m, 2H), 7.82 (s , 2H), 7.96 (s, 1H).
Embodiment 3
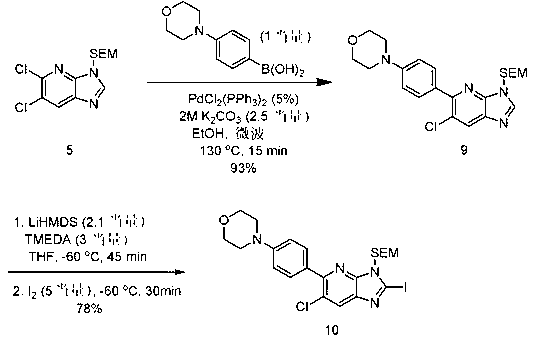
[1184] 【Chemical 72】
[1185]
[1186] Add 4-morpholinophenylboronic acid (2.75 g, 13.29 mmol), PdCl 2 (PPh 3 ) 2 (467 mg, 0.67 mmol), potassium carbonate aqueous solution (2 M, 16.6 ml, 33.23 mmol), stirred at 130°C for 15 minutes under microwave irradiation. Water and ethyl acetate were added to the reaction suspension for extraction, and the organic layer was washed with water and saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography to obtain Compound 9 (5.51 g, 93%) as a yellow solid.
[1187] Compound 9; 1 H-NMR (DMSO-d 6) δ: 0.00 (s, 9H), 0.97 (t, J = 8.0 Hz, 2H), 3.32 (t, J = 4.8 Hz, 4H), 3.74 (t, J = 8.0 Hz, 2H), 3.88 (t, J = 4.8 Hz, 4H), 5.75 (s, 2H), 7.15 (d, J = 8.4 Hz, 2H), 7.73 (d, J = 8.4 Hz, 2H), 8.45 (s, 1H) , 8.78 (s, 1H).
[1188] TMEDA (4.8 ml, 31.70 mmol) was added to compound 9 (4.7 g, 10.56 mmol) in anhydrous THF (47 mL), and a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com