Medicine composition containing myricetrin or/and myricetin and application of medicine composition in preparation of medicine used for treating Parkinson
A composition and a technology for Parkinson's disease are applied in a pharmaceutical composition containing salicylic glycoside or/and aglycone myricetin to prepare medicines for treating Parkinson's disease, can solve problems such as liver damage, achieve good anti-inflammatory, Good antioxidant activity, high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
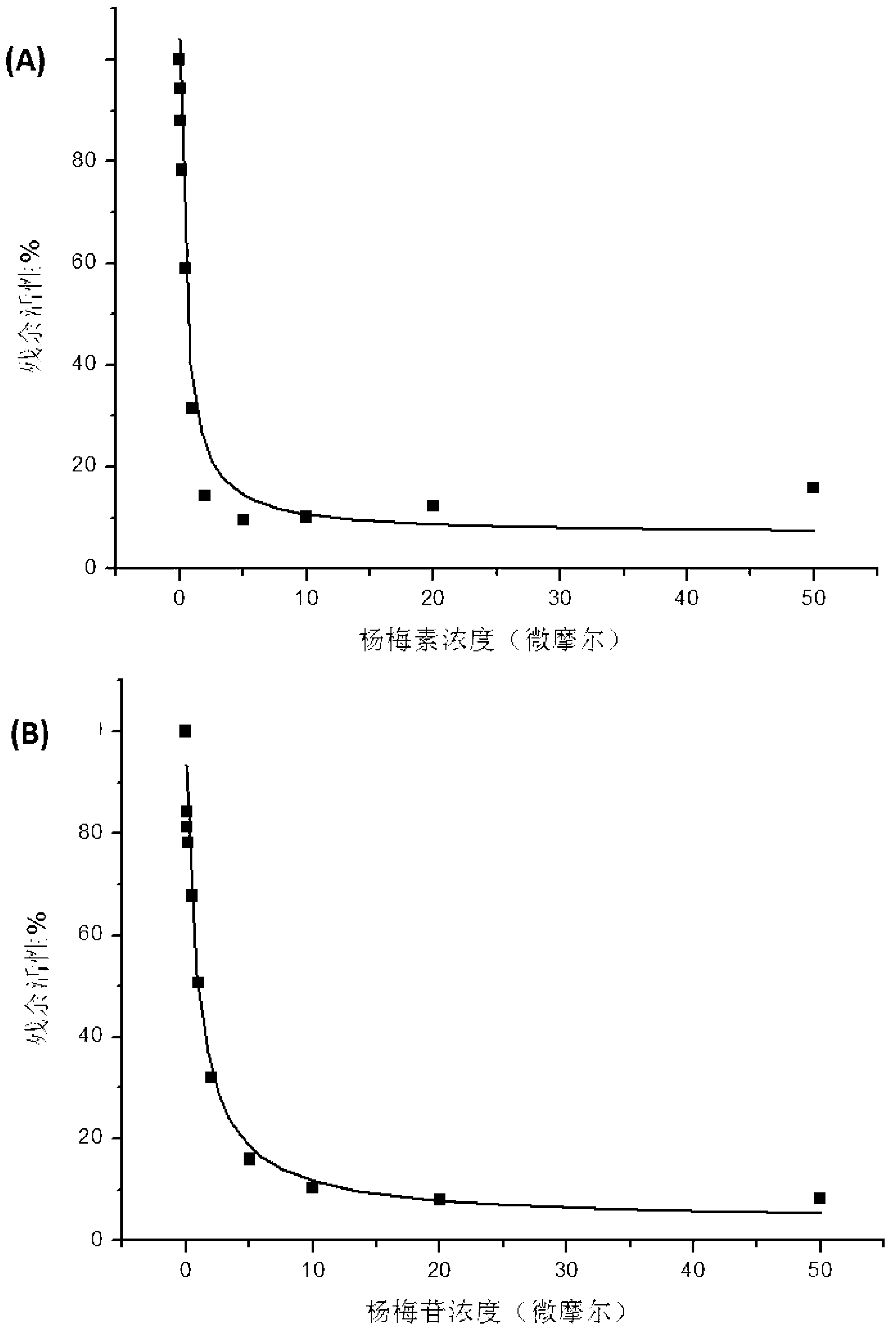
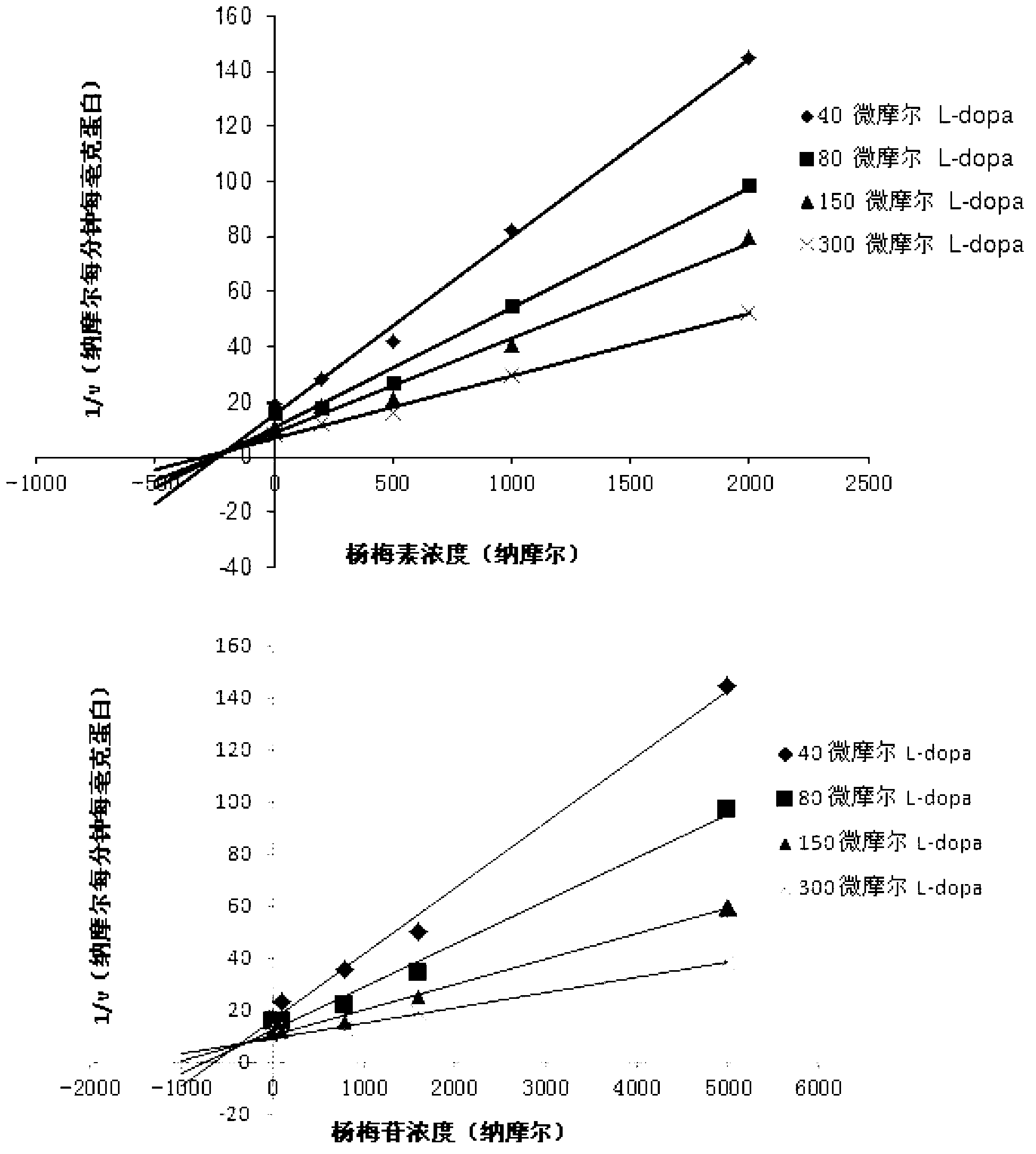
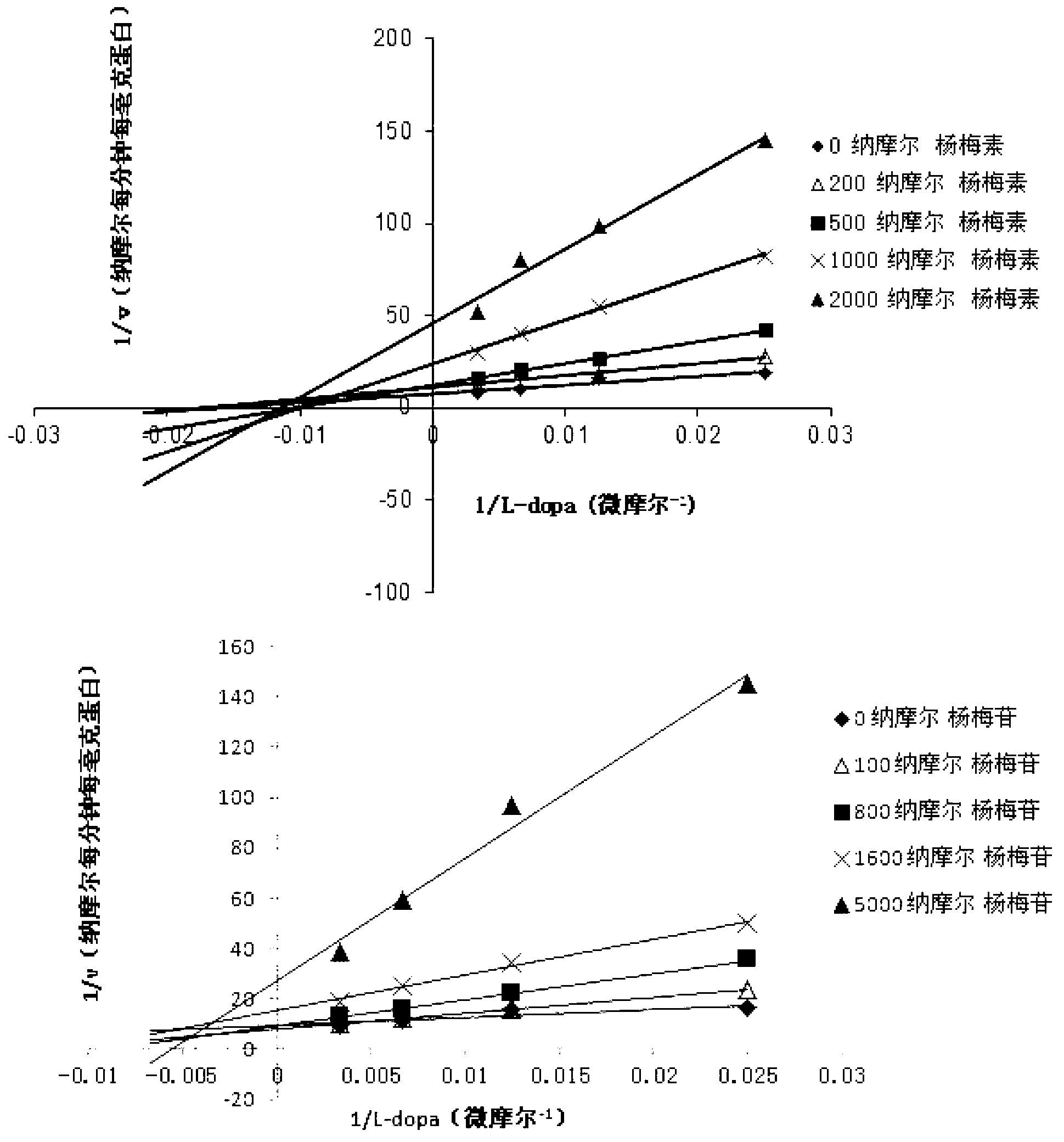
[0031] Example 1 Determination of Inhibitory Activity of Myricetin and Its Aglycone Myricetin Monomer to Human COMT Enzyme
[0032] Using the L-dopa methylation reaction as a probe reaction, the IC of myricetin and its aglycon myricetin on the inhibition of COMT enzyme was determined by means of the in vitro incubation system of human hepatocytes 50 , the specific experimental procedure is as follows:
[0033] (1) Add 5mM MgCl to 200 microliters of in vitro metabolic reaction system 2 , 2mM dithiothreitol, 150μM substrate L-dopa, human liver cell plasma protein concentration is 1mg / ml, inhibitor final concentration range is 0.05μM-50μM, pre-incubated at 37℃ for 3 minutes;
[0034] (2) Add S-adenosylmethionine (final concentration: 0.2mM) to the reaction system to initiate the reaction; after reacting at 37°C for 30 minutes, add 200 μl of acetonitrile, shake vigorously, and terminate the reaction;
[0035] (3) Using a high-speed refrigerated centrifuge, under the condition of 2...
Embodiment 2
[0037] Example 2 Toxicity Evaluation of Myricetin and Its Aglycone Myricetin
[0038] Select Kunming mice, weighing 178-22g. The mice were divided into random groups, 20 in each group, half male and half male, and the oral acute toxicity test of myricetin and myricetin were carried out in mice respectively. Myricetin or myricetin were suspended in 0.5% CMC-Na, respectively. Select different doses of oral administration (0.1 ~ 2g / kg); and set 0.5% CMC-Na group as a negative control. Experimental results show that myricetin and myricetin oral administration of LD in mice 50 The value is greater than 2.0g / kg, which belongs to the non-toxic level. In addition, in vitro cell experiments confirmed that myricetin and myricetin had no obvious toxicity to primary human liver cells and kidney cells. At the same time, the myricetin and its aglycone myricetin (final concentration: 100 μM) and reduced glutathione (final concentration: 1000 μM) were respectively added to the human liver...
Embodiment 3
[0039] Example 3 Pharmacokinetic study of myricetin and its aglycon myricetin combined with levodopa
[0040] As a COMT enzyme inhibitor, myricetin or / and myricetin has no therapeutic effect on Parkinson's disease when administered alone, and it is meaningful only when combined with levodopa.
[0041] Select 24 Wistar rats, half male and half female, body weight 180-220g, randomly divided into 4 groups: oral levodopa / carbidopa control group, oral myricetin / levodopa / carbidopa group (myricetin The molar ratio of myricetin to levodopa is 5:1), oral administration of myricetin / levodopa / carbidopa group (the molar ratio of myricetin to levodopa is 5:1), oral administration of myricetin-myricetin mixture / levodopa In the ba / carbidopa group (the molar ratio of the sum of the moles of myricetin and myricetin to levodopa is 5:1), 6 animals / group were used to carry out the overall pharmacokinetic study of L-dopa. The oral dose is 2g / kg. About 0.5ml of rat plasma samples were collected b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com