Preparation method of N-carbobenzoxy-3-amino propionaldehyde
A technology of benzyloxycarbonyl and aminopropanal, applied in the field of preparation of N-benzyloxycarbonyl-3-aminopropanal, a drug synthesis intermediate, can solve the problems of inapplicability to large-scale production, unfavorable amplification reaction, unfriendly environment, etc. , to achieve the effect of easy purification, environmental friendliness and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
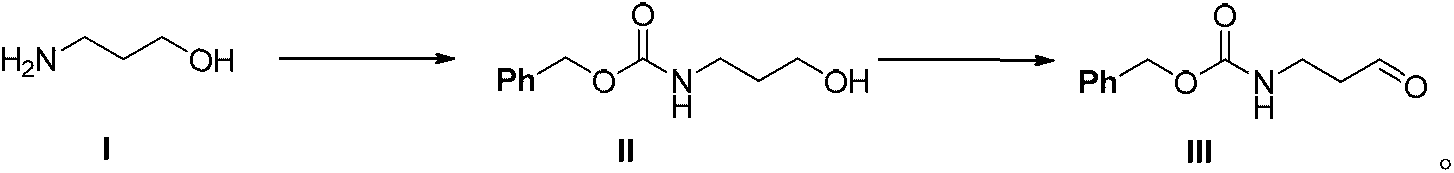
[0021] A kind of preparation method of N-benzyloxycarbonyl-3-aminopropanal of the present invention, take 3-aminopropanol as starting material, obtain 3-(benzyloxycarbonylamino)-1-propanol through amidation reaction; Then , through the oxidation reaction of sodium hypochlorite, 2,2,6,6-tetramethylpiperidine oxide and potassium bromide under the condition of pH 8.0-9.5, to obtain the N-benzyloxycarbonyl-3-aminopropanal. Specifically include the following:
[0022] 1) Amidation reaction: 3-aminopropanol (I) and benzyl chloroformate are placed in triethylamine and methylene chloride solution for amidation reaction to obtain 3-(benzyloxycarbonylamino)-1-propanol (II). The amidation reaction is carried out at 0-10°C. Further, after the amidation reaction is completed, the reaction solution is washed with 1M HCl and brine in sequence, and the obtained organic phase is directly used for the next oxidation reaction.
[0023] 2) Oxidation reaction: the oxidation reaction is carried ...
Embodiment 1
[0024] Embodiment 1 Amidation reaction prepares 3-(benzyloxycarbonylamino)-1-propanol (II)
[0025] 3-Aminopropanol (82.6g), triethylamine (111.3g) and dichloromethane (1L) system was cooled to 0°C, and benzyl chloroformate (170.5g) was slowly added dropwise to the system, and the dropwise addition process Maintain the temperature at 0-10°C; continue to react at this temperature for 2h. After liquid separation, the organic phase was washed with 1M hydrochloric acid and brine in sequence, and was directly used in the next reaction (see Example 2).
Embodiment 2
[0026] Embodiment 2 oxidation reaction prepares N-benzyloxycarbonyl-3-aminopropanal (III)
[0027] A dichloromethane solution of 3-(benzyloxycarbonylamino)-1-propanol prepared in Example 1, 2,2,6,6-tetramethylpiperidine oxide (1.56 g) and dichloromethane ( 600ml) system was cooled to 0°C, and potassium bromide (17.85g) was dissolved in water (100-200ml) and added to the aforementioned reaction system. Then, slowly drop sodium hypochlorite solution (active chlorine 10-14.5%, 2 equivalents) and sodium bicarbonate (46.28g)-aqueous solution (pH8.0-9.5) in the system, and the dropping process control temperature is not high At 10 °C, the reaction was continued at this temperature for 1 h. The liquid was separated, and the organic phase was successively washed with 1M hydrochloric acid-potassium iodide solution, 10% sodium thiosulfate solution, 5% sodium bicarbonate solution and brine, and dried over anhydrous sodium sulfate. Concentration gave an off-white solid (173.8 g, purity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com