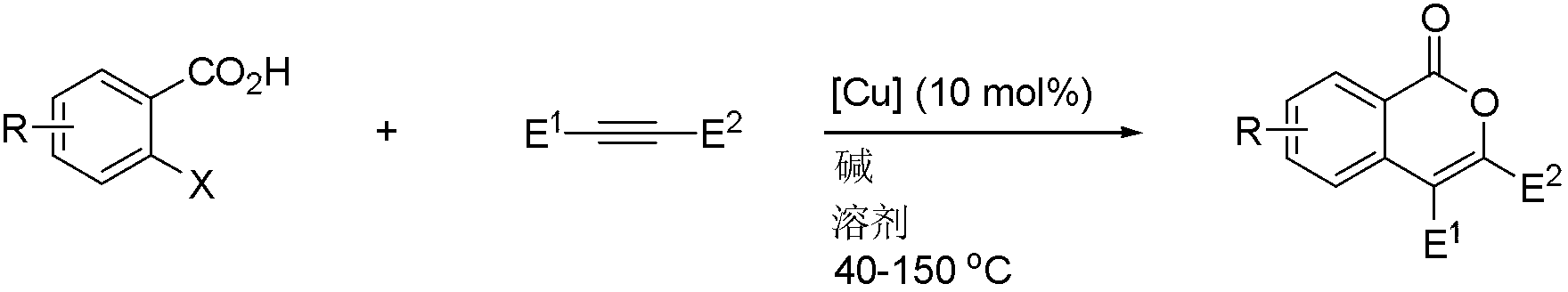
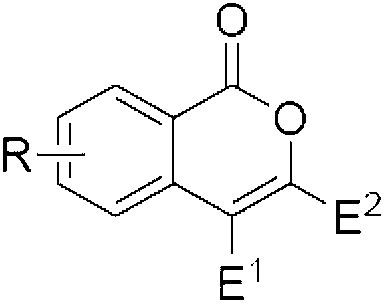
Isocoumarin compound, derivatives and synthesis method thereof
A technology of isocoumarin and synthesis method, applied in the field of isocoumarin, can solve the problems of harsh reaction conditions, increased cost and the like, and achieves the effects of low cost, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
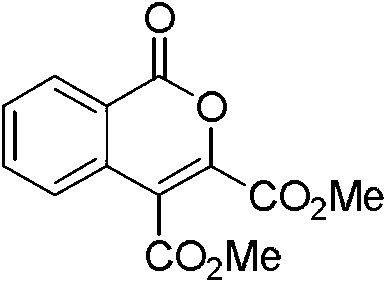
[0025] 3,4-dimethoxycarbonyl isocoumarin, its structural formula is:
[0026]
[0027] The synthetic method of this compound is as follows:
[0028] In a reaction tube, add 4.0mg cupric chloride, 74.4mg 2-iodobenzoic acid, 85.2mg dimethyl butyndioate, 82.9mg potassium carbonate, and 2.0mL toluene under nitrogen protection, and slowly heat up from room temperature to 130°C, react for 12 hours. Conventional treatment yielded 64.5 mg of pure product with a yield of 82%.
[0029] The melting point of the product is 132-133°C; the result of NMR characterization is: 1 H NMR (400MHz, CDCl 3 )δ8.36(d, J=8.6Hz, 1H), 7.84(d, J=8.6Hz, 1H), 7.70(d, J=8.6Hz, 1H), 7.54(d, J=8.6Hz, 1H) , 4.01(s, 3H), 3.96(s, 3H); 13 C NMR (CDCl 3 )δ 53.5 (2C), 119.1, 122.0, 125.6, 130.4, 131.4, 132.8, 135.7, 140.9, 159.3, 160.2, 165.1. The above results indicated that the desired product was synthesized.
Embodiment 2
[0031] 5-methyl-3,4-dimethoxycarbonyl isocoumarin, its structural formula is:
[0032]
[0033] The synthetic method of this compound is as follows:
[0034] In a reaction tube, add 4.0mg cupric chloride, 78.6mg 3-methyl-2-iodobenzoic acid, 85.2mg dimethyl butyndioate, 82.9mg potassium carbonate, and 2.0mL toluene under nitrogen protection, from room temperature Start to slowly heat up to 130°C, and react for 12 hours. Conventional treatment yielded 54.7 mg of pure product with a yield of 66%.
[0035]The melting point of the product is 144-145°C; the result of NMR characterization is: 1 H NMR (400MHz, CDCl 3 )δ8.31(d, J=8.0Hz, 1H), 7.55-7.63(m, 2H), 4.01(s, 3H), 3.96(s, 3H), 2.51(s, 3H); 13 C NMR (CDCl 3 )δ20.1, 53.4 (2C), 118.9, 123.2, 129.1, 130.9, 131.1, 136.2, 139.1, 140.4, 159.9, 160.5, 167.0; high-resolution mass spectrometry HRMS (ESI) calculated as: C 14 h 13 o 6 , 277.0712(M+H) + , found: 277.0706. The above results indicated that the desired product was...
Embodiment 3
[0037] 7-methyl-3,4-dimethoxycarbonyl isocoumarin, its structural formula is:
[0038]
[0039] The synthetic method of this compound is as follows:
[0040] In a reaction tube, under nitrogen protection, add 4.0mg copper chloride, 78.6mg 5-methyl-2-iodobenzoic acid, 85.2mg dimethyl butynedioate, 82.9mg potassium carbonate, and 2.0mL toluene, from room temperature Start to slowly heat up to 130°C, and react for 12 hours. Conventional treatment yielded 57.9 mg of pure product with a yield of 70%.
[0041] The melting point of the product is 164-165°C; the result of NMR characterization is: 1 H NMR (400MHz, CDCl 3 )δ8.18(s, 1H), 7.65(d, J=8.0Hz, 1H), 7.42(d, J=8.0Hz, 1H), 4.02(s, 3H), 3.96(s, 3H), 2.52( s, 3H); 13 CNMR (CDCl 3 )δ21.7, 53.4, 53.5, 119.3, 121.9, 125.5, 130.3 (2C), 136.8, 140.1, 142.4, 159.5, 160.3, 165.3; high resolution mass spectrometry HRMS (ESI) calculated as: C 14 h 13 o 6 (M+H) + , 277.0712, found: 277.0699. The above results indicated that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com