Composition of statin medicine and 5-methyltetrahydrofolic acid, and application thereof
A technology of methyltetrahydrofolate and a composition, applied in the field of pharmacy, can solve the problems of elevated transaminase and the like, and achieve the effects of reducing the elevation of transaminase, improving compliance, and being convenient for taking medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
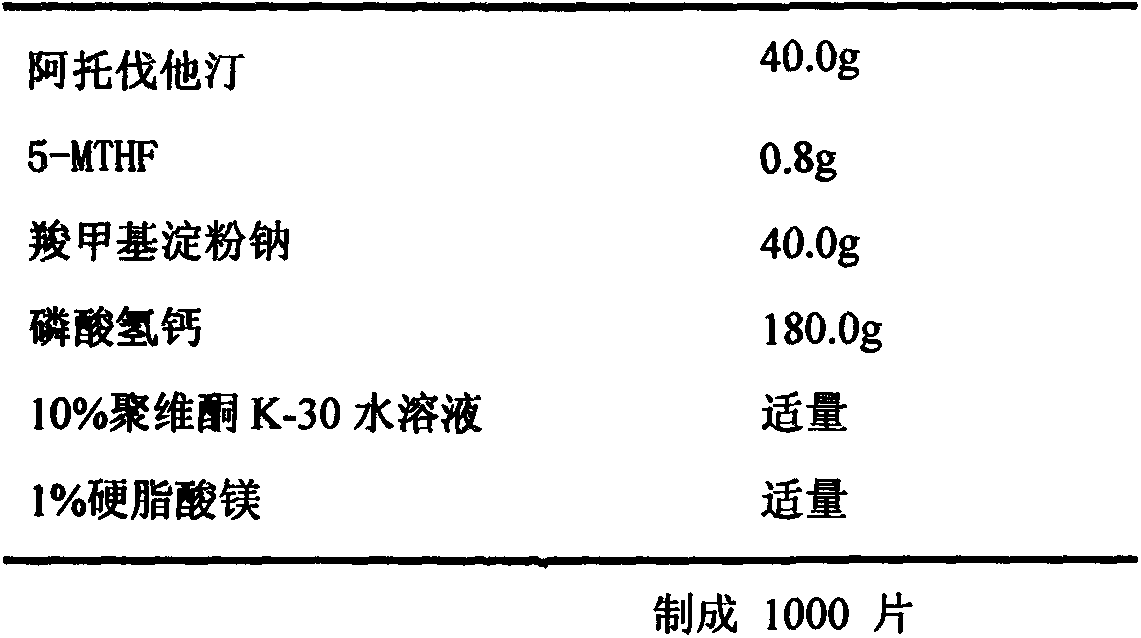
[0035] Embodiment 1. Preparation of atorvastatin 40mg / 5-methyltetrahydrofolate 0.8mg tablet
[0036] Recipe composition:
[0037]
[0038] Preparation method: crush the raw and auxiliary materials through an 80-mesh sieve, and dry for later use. Take 40g of atorvastatin and 0.8g of 5-methyltetrahydrofolate and mix evenly according to the method of equal increase, add 40g of sodium carboxymethyl starch and 180g of calcium hydrogen phosphate, mix evenly according to the method of equal increase, and add an appropriate amount of binder 10% povidone aqueous solution to make soft material, granulate with 30 mesh, dry at 40-45°C for 3 hours; granulate with 30 mesh, finally add appropriate amount of 1% magnesium stearate and mix evenly, after content determination, compress into tablets and pack. Pay attention to avoiding light during the preparation process. After the finished product passes the inspection, it will be packaged in aluminum-plastic blisters and stored away from li...
Embodiment 2
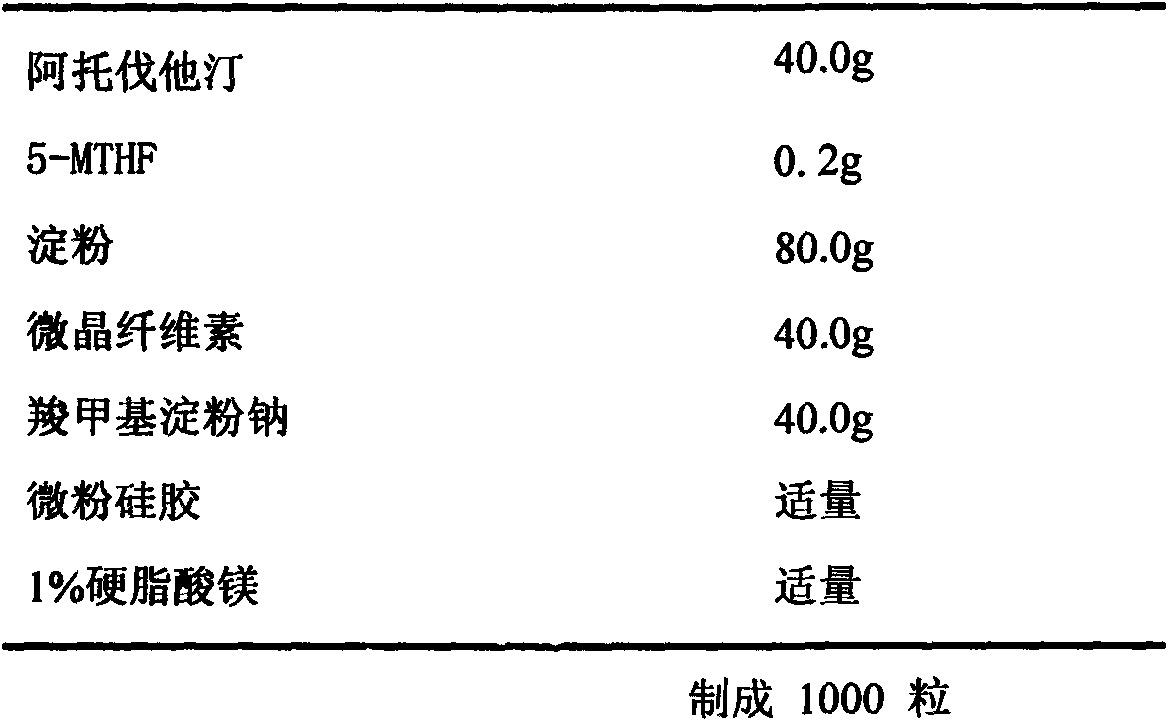
[0039] Example 2. Preparation of Atorvastatin 40mg / 5-Methyltetrahydrofolate 0.2mg Capsules
[0040] Recipe composition:
[0041]
[0042] Preparation method: crush the raw and auxiliary materials through an 80-mesh sieve, and dry for later use. Take 40g of atorvastatin and 0.2g of 5-methyltetrahydrofolate and mix uniformly according to the method of equal increase, add 80g of starch, 40g of microcrystalline cellulose, and 40g of sodium carboxymethyl starch, and mix uniformly according to the method of equal increase. Micro-powdered silica gel is made into a soft material, granulated with a 20-mesh sieve, dried at 60°C for about 2 hours, granulated with a 20-mesh sieve, and the moisture content of the granules is controlled to 2-3%, and the dried granules are mixed with 1% stearic acid in the prescribed amount The magnesium is mixed evenly, the semi-finished product is tested, the content is measured, and the hollow capsules are filled to obtain 1000 capsules. Protect from...
Embodiment 3
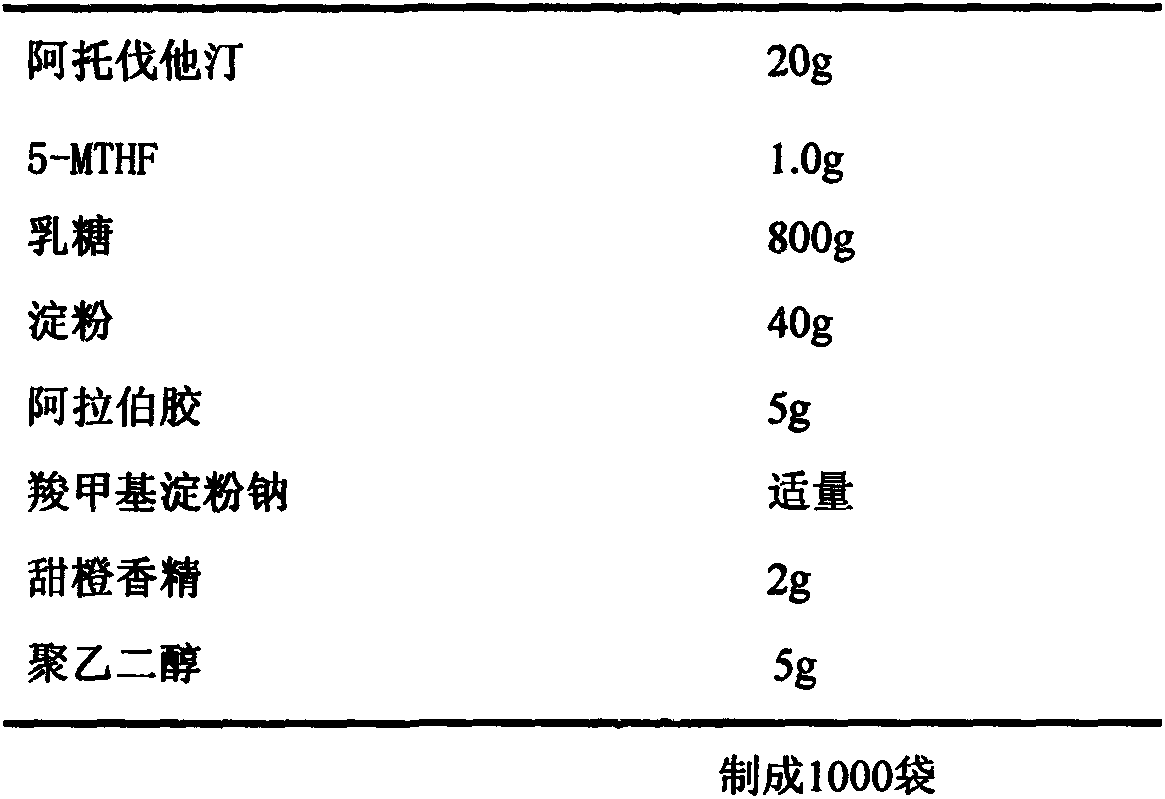
[0043] Example 3. Preparation of atorvastatin 20mg / 5-methyltetrahydrofolate 1.0mg granules
[0044] Recipe composition:
[0045]
[0046] Preparation process: Preparation process: crush the raw materials and auxiliary materials through a 120-mesh sieve, take 20 g of atorvastatin, 1.0 g of 5-methyltetrahydrofolate, 800 g of lactose, 40 g of starch, 5 g of gum arabic, 2 g of sweet orange essence, polyethylene glycol Glycol 5g is crushed and sun-dried, mixed evenly to make soft material, granulated, dried at 40-60°C, water content is about 3%, taken out, granulated, added appropriate amount of sodium carboxymethyl starch and mixed evenly, according to the conventional method It can be filled into granules. Each bag of the prepared compound tablet contains 20 mg of atorvastatin and 1.0 mg of 5-methyltetrahydrofolate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com