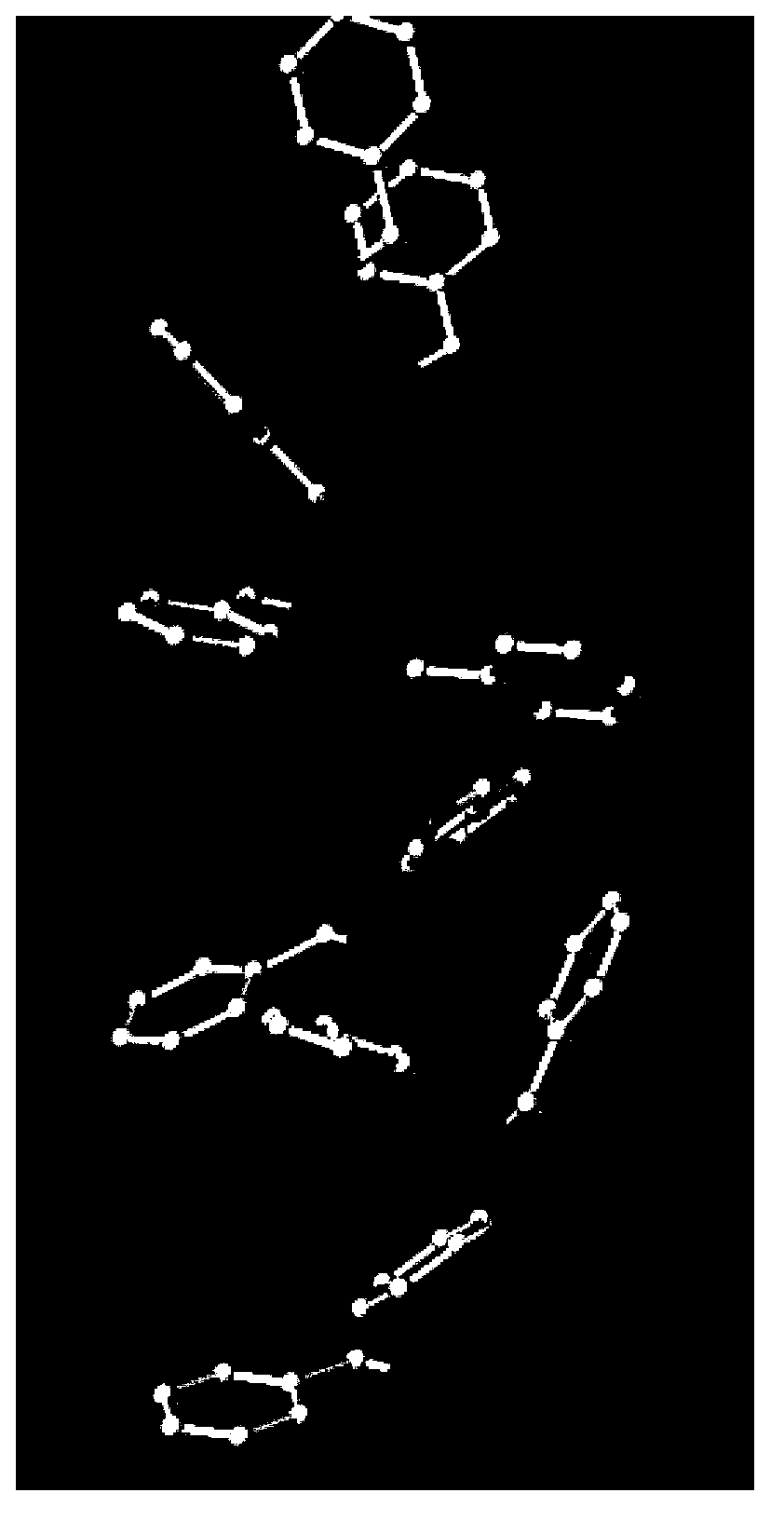
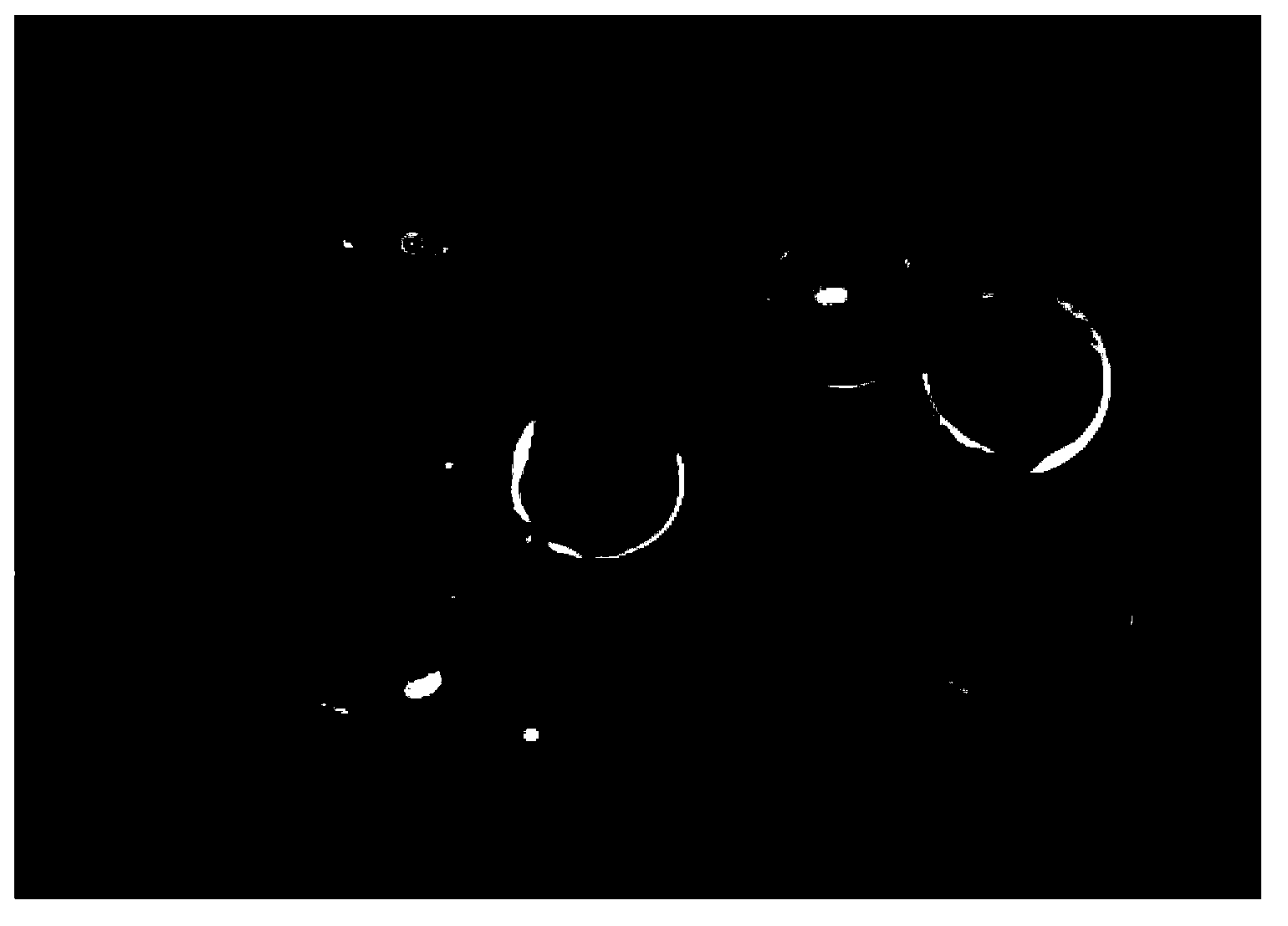Heparin-phenylalanine adsorption material for blood purification method for removing endotoxin
A technology of blood purification and phenylalanine, which is applied in the field of biomedical engineering, can solve the problems of death of Gram-negative bacteria and no safe and effective specific drugs have been found, and achieve a high removal rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 heparin-phenylalanine adsorption material
[0037] (1) Amination of chloromethyl resin
[0038] Take the chloromethyl resin and wash it alternately with absolute ethanol and distilled water three times each, and drain it with a suction filter pump for later use; place 5-10 g of the obtained chloromethyl resin in 20-60 ml of 1,4-dioxane to swell for 2-6 hours, Transfer to a three-necked flask; dissolve 0.1-1g of tetrabutylammonium bromide and 1-10g of NaOH in 40ml of distilled water and add to the three-necked flask; then add 10-50ml of ethylenediamine to the three-necked flask; control the reaction The temperature is 40-100°C, and the reaction is stirred for 1-9 hours; the product obtained from the reaction is filtered, and the filter residue is alternately washed with absolute ethanol and distilled water until neutral, and finally dried in vacuum to obtain an aminated chloromethyl resin;
[0039] (2) Connection of spacer arm heparin
[0...
Embodiment 2
[0043] Example 2 Observation of the adsorption of fluorescent endotoxin (FITC-LPS) by heparin-phenylalanine adsorption material under fluorescence microscope
[0044] Adsorption experiments were carried out on 0.2 g of the heparin-phenylalanine adsorption material prepared in Example 1 in rabbit plasma solutions containing 50 μg / mL fluorescent endotoxin. The adsorption reaction time was 2 hours, and the surface fluorescence intensity was observed by an inverted fluorescence microscope. Qualitatively observe the adsorption situation; under the same experimental conditions, the aminated chloromethyl resin prepared in Example 1 step (1) and the heparinized chloromethyl resin prepared in Example 1 step (2) were used as a control group, and the experimental results were as follows: Figure 2~4 shown. figure 2 It is the effect diagram of heparin-phenylalanine adsorption material adsorbing fluorescently labeled endotoxin, image 3 is the effect diagram of heparinized chloromethyl r...
Embodiment 3
[0045] Example 3 Heparin-phenylalanine adsorption material is used for the removal of endotoxin in rabbit plasma
[0046] Limulus reagent chromogenic matrix method is used for the quantitative detection of endotoxin. The basic principle is that bacterial endotoxin reacts enzymatically with factor C in Limulus reagent to produce coagulation enzyme, which can decompose the artificially synthesized chromogenic matrix into yellow paranitrate Phenylaniline (PNA) and peptides. PNA can be further dyed rose color by azo reagent, and the absorbance value in the reaction solution can be measured at 545nm for endotoxin quantification.
[0047] Take 0.2 g of the heparin-phenylalanine adsorption material prepared in Example 1 and fill it into a 2 mL syringe, plug both ends with absorbent cotton, connect a constant-flow pump and a beaker to make a hemoperfusion device for dynamic plasma perfusion experiments.
[0048] The Limulus kit was used to detect the adsorption amount of the heparin-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com