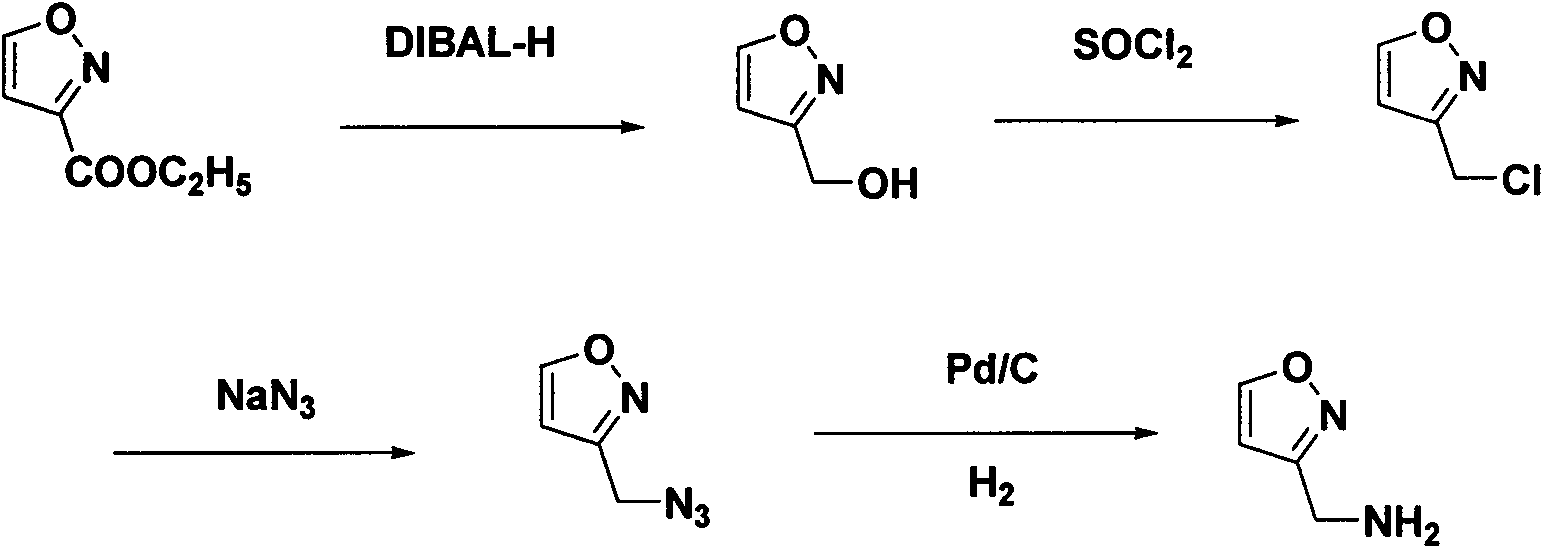
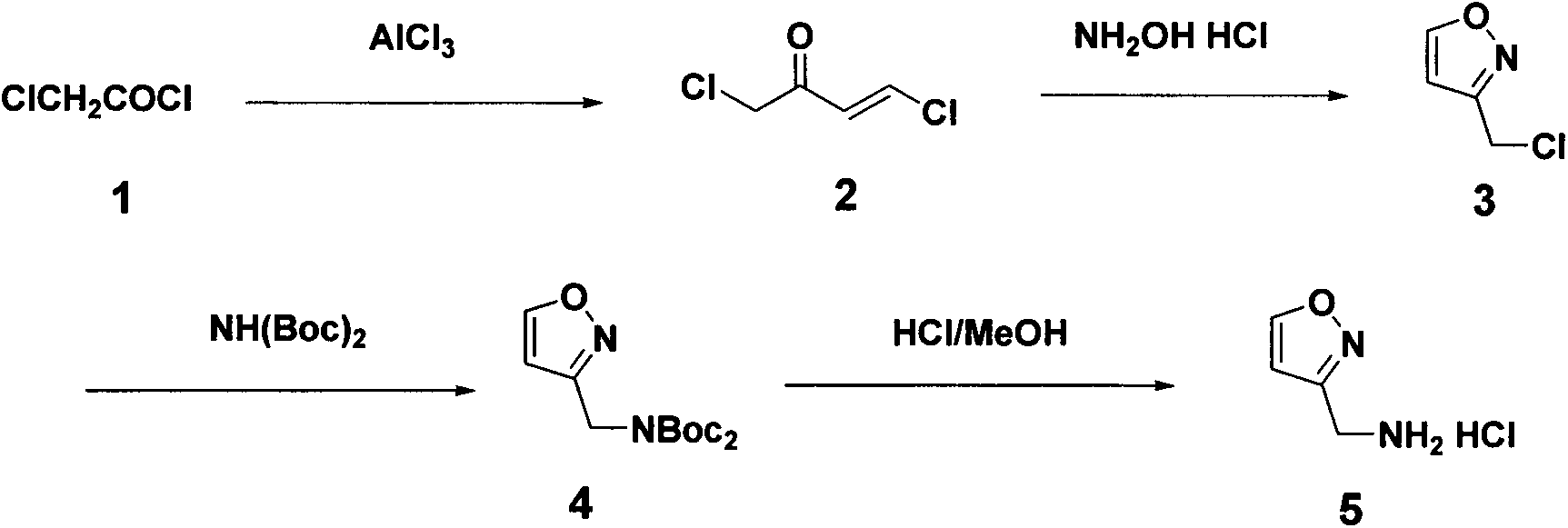
Synthetic method of 3-aminomethyl-isoxazole hydrochloride
A technology of isoxazole hydrochloride and synthesis method, which is applied in the fields of chemical industry and medicine, can solve the problems of not being suitable for large-scale production, high cost, difficult preparation of starting materials, etc., and achieve shortened production process, mild conditions and high product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
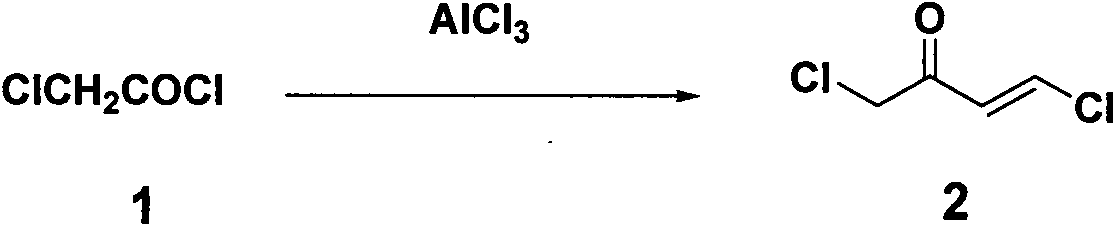
[0019] Embodiment 1: Synthesis of 2,4-dichloro-2-butenone (compound 2)
[0020] Chloroacetyl chloride (120 g, 1.06 mol) was slowly added dropwise to anhydrous AlCl 3 (156g, 1.17mol) and CCl 4 (320mL), the mixture was cooled to -5°C, acetylene gas was introduced for 2 hours, the reactant was slowly poured into ice water, the organic layer was washed with cold water, dried over anhydrous sodium sulfate, and collected under reduced pressure at 78-83°C / 16mmHg The distillate obtained 65 g of oily product 2,4-dichloro-2-butenone, yield: 44.2%.
Embodiment 2
[0021] Embodiment 2: Synthesis of 3-chloromethyl-isoxazole (compound 3)
[0022] 2,4-Dichloro-2-butenone (50g, 0.36 mol), methanol (200mL) and hydroxylamine hydrochloride (25g, 0.36 mol), heated to reflux for 4 hours, distilled methanol under reduced pressure, Na 2 CO 3 The solution was adjusted to alkaline, extracted with ethyl acetate, washed with water, anhydrous Na 2 SO 4 Dry and distill under reduced pressure to obtain 26 g of oily 3-chloromethyl-isoxazole, yield: 61.5%.
[0023] 1 H-NMR (CDCl 3 , 300MHz): δ8.39(d, 1H), 6.46(d, 1H), 4.62(s, 2H).
Embodiment 3
[0024] Embodiment 3: Synthetic bis-Boc aminomethyl-isoxazole (compound 4)
[0025] 3-Chloromethyl-isoxazole (24g, 0.20mol), NH(Boc) 2 (43.4g, 0.20mol), K 2 CO 3 (27.6g, 0.20 mol), tetrabutylammonium bromide (1.6g, 0.005 mol) and acetonitrile (80mL), stirred and refluxed overnight, cooled, added water (250mL), extracted with ethyl acetate, anhydrous Na 2 SO 4 Dry and concentrate to obtain 42 g of oily bis-Boc aminomethyl-isoxazole, yield: 70.5%.
[0026] 1 H-NMR (CDCl 3 , 300MHz): δ8.33(d, 1H), 6.29(d, 1H), 4.87(s, 2H), 1.47(s, 18H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com