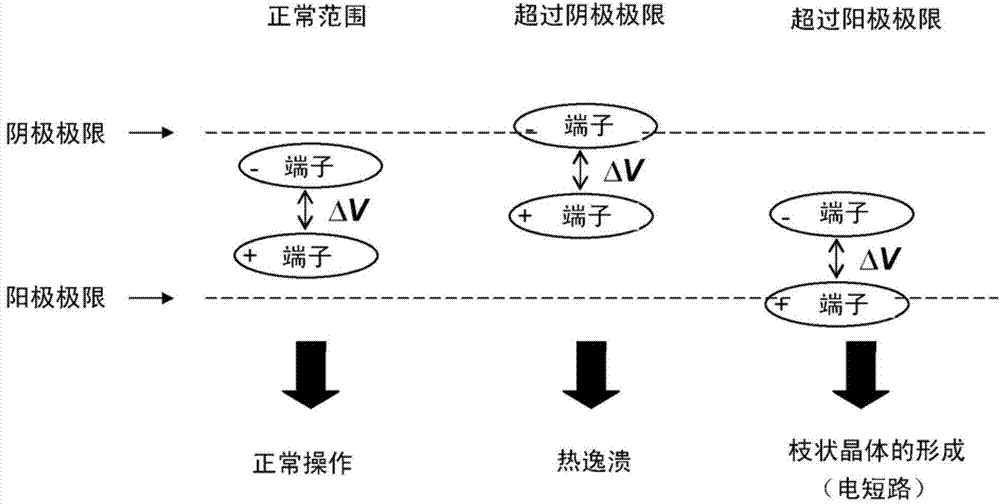
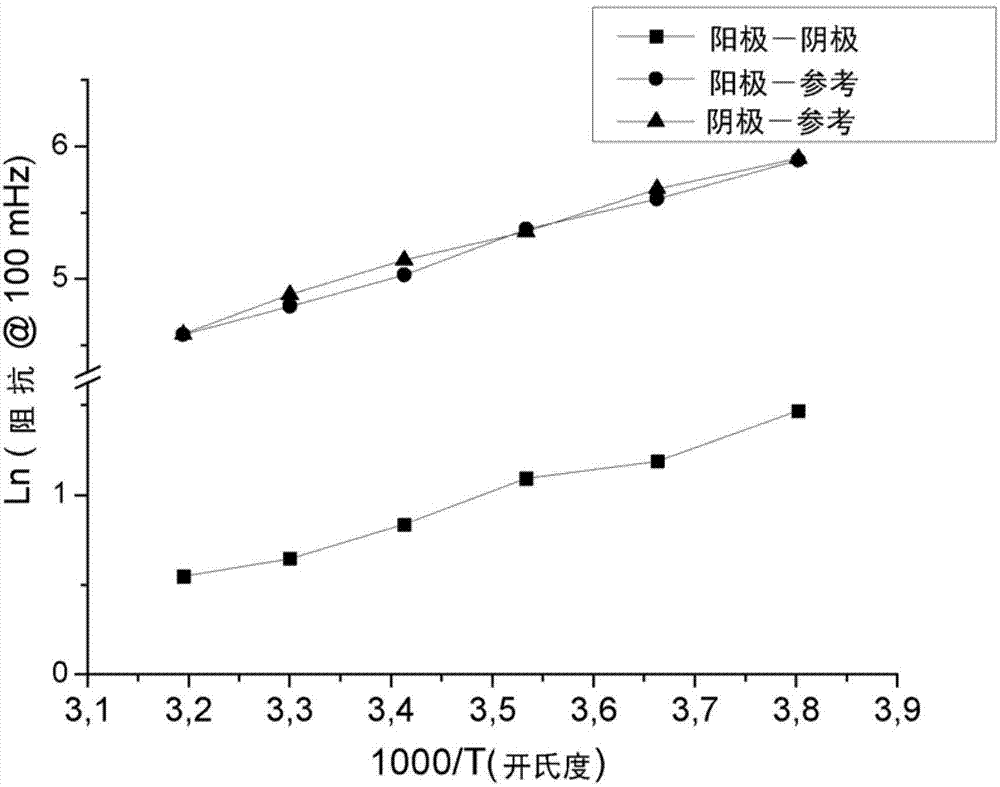
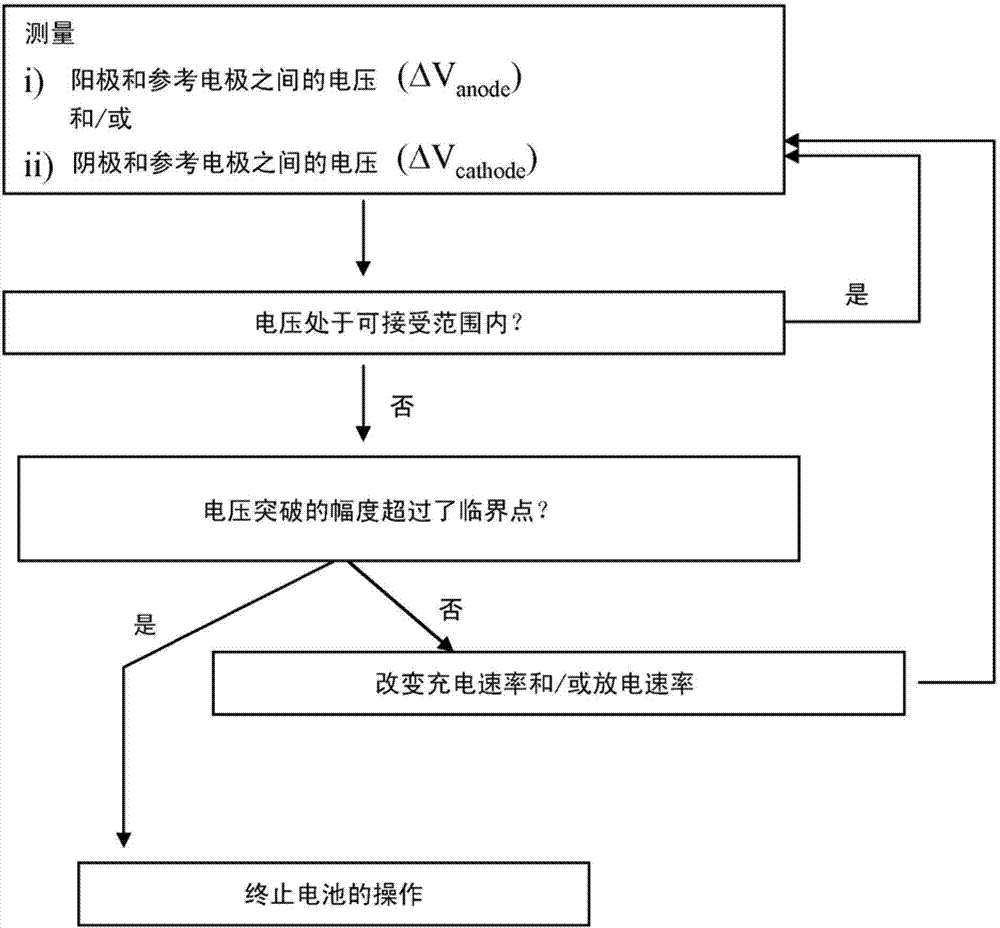
Electrochemical cell based on lithium technology with internal reference electrode, process for its production and methods for simultaneous monitoring of the voltage or impedance of the anode and the cathode thereof
A reference electrode, electrochemical technology, used in secondary battery manufacturing, lithium storage batteries, electrochemical generators, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0186] Example 1: Preparation of electrodes
[0187] Positive electrode: Lithium cobalt oxide containing cathode material (LiCoO 2 ) of the positive electrode. The casting paste is made of LiCoO 2 , graphite, carbon black, and PVDF (Kynar LBG2) as a binder are composed of homogeneous mixtures formed in acetone at a rate of 90%, 2.5%, 2.5%, and 5%, respectively. Casting of the slurry was done using a doctor blade on a plastic film resting on a flat glass substrate. After evaporation of the solvent, the specific capacity of the cathode was determined to be 3.15 mAh / cm 2 And has a thickness of 208 μm. The electrode material was laminated onto an aluminum current collector having a thickness of 120 μm.
[0188] Negative electrode: The negative electrode containing the anode material graphite is prepared by a casting process. The casting slurries consisted of homogeneous mixtures of graphite, carbon black and PVDF (Kynar LBG2) as a binder in acetone at ratios of 85%, 5% and 1...
example 2
[0190] Example 2: Characterization of electrode materials
[0191] Linear sweep voltammetry (LSV) or cyclic voltammetry (CV) was performed on the three types of electrodes prepared according to Example 1. All experiments were carried out in electrochemical cells with lithium metal foil serving as reference and counter electrode. The electrolyte used is composed of 1:1 ethylene carbonate (EC) and contains 1M LiPF 6 A battery-grade mixture of dimethyl carbonate (DEC). The electrode area of the working electrode (that is, the cut circle of the electrode foil in Example 1) is 1.3cm 2 . All experiments were performed under an argon atmosphere. Use slow scan rates to better resolve voltammetric peaks. Specifically, for graphite and Li 4 Ti 5 o 12 Both use 10μV / s, for LiCoO 2 Use 100 μV / s to obtain the voltammogram of the electrode ( Figure 7-9 ).
[0192] In LiCoO 2 In the case of , 3 cycles were performed to verify the evolution of the voltammetric peak with the help...
example 3
[0196] Example 3: Full cell design 1 and its construction
[0197] Using the electrode materials prepared as described in Example 1 and containing Li at a ratio of 75% and 25%, respectively 1.3 Al 0.3 Ti 1.7 (PO 4 ) 3 and PVDF spacers to construct full cells. The thickness of the spacer was 55 μm. Figure 10 A battery construction scheme of an embodiment of the present invention is shown.
[0198] The cathode material was laminated onto an aluminum current collector having a thickness of 120 μm. The anode was laminated onto a copper current collector with a thickness of 120 μm. Both the cathode and anode have external dimensions of 58 x 33 mm with an 8 x 5 mm notch cut from the center of one side inwards of the electrode. The separator has slightly larger dimensions compared to the anode and cathode. The reference electrode material was laminated onto a copper current collector with a thickness of 120 μm. Reference electrodes for batteries are obtained by cutting strip...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com