Antimicrobial peptide with drug-resistance bacteria resistance activity and synthesis and application thereof
An antibacterial peptide and drug-resistant bacteria technology, applied in the field of biochemistry, can solve the problems of natural antibacterial peptides are not very active and have a narrow antibacterial spectrum.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
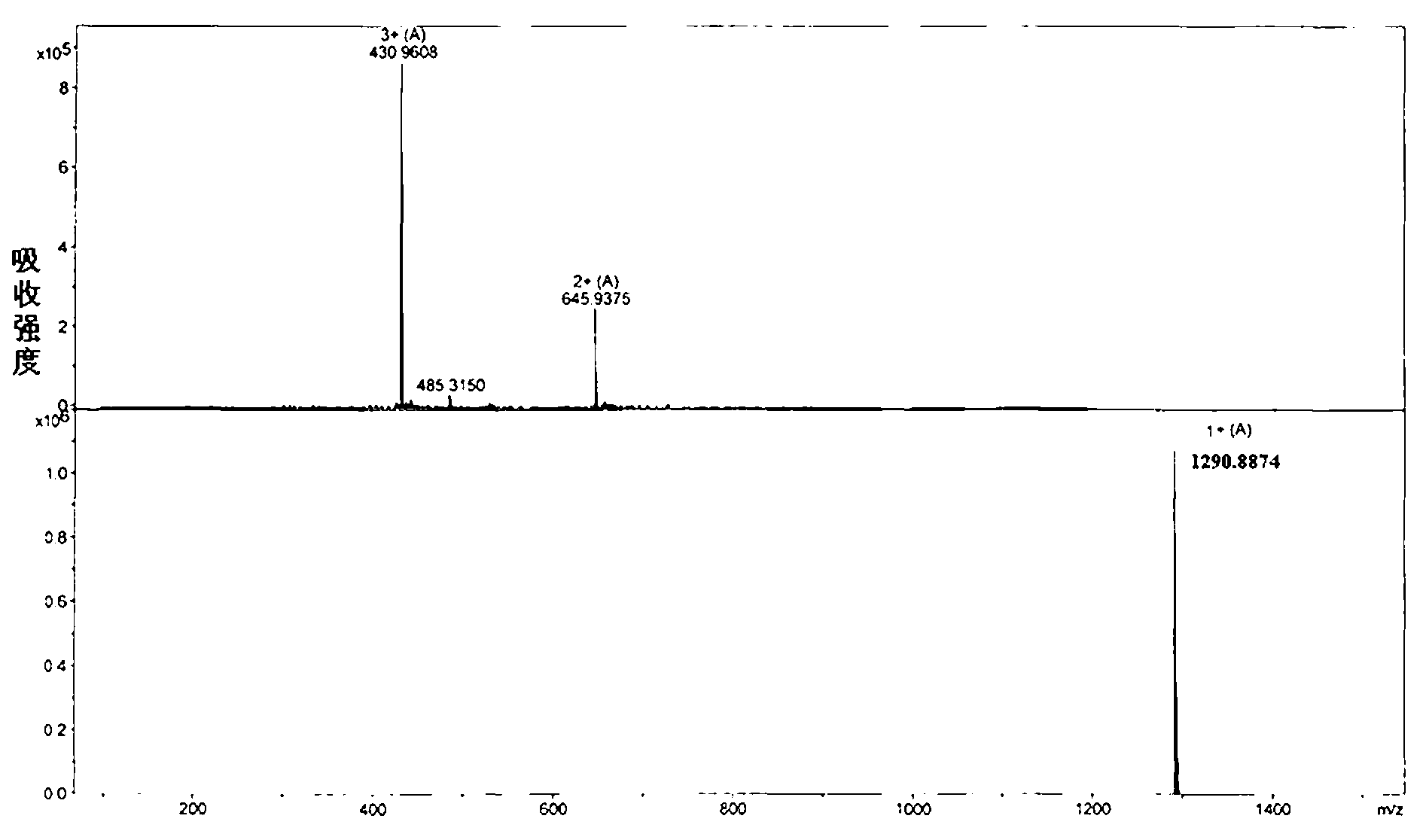
[0072] Embodiment 1: the synthesis of antimicrobial peptide J-AM
[0073] (1) Activation and pretreatment of resin
[0074] Accurately weigh 0.7g of MBHA resin (0.43mmol / g), add it to the peptide solid-phase synthesizer, add DCM along the tube wall until the resin is completely immersed, stir for 30 minutes to fully swell the resin, and then remove the stirrer, DCM was aspirated (30 min).
[0075] (2) Synthesis of AC-pra-Anoplin-MBHA
[0076] a. Leu coupling: Wash the pretreated resin three times with DMF, each time for 3 min (2 min×3 times), use a vacuum pump to dry the solvent in the reactor, add 20% volume fraction of hexahydropyridine / DMF solution, stirred (2 min×4), and drained. After the reaction was completed, wash with DMF 3-4 times, 2 min each time. Test whether the Fmoc protecting group has been removed by ninhydrin chromogenic method: if it is colorless, the Fmoc protecting group has not been removed, repeat the above steps; if it is blue, the Fmoc protecting gr...
Embodiment 2
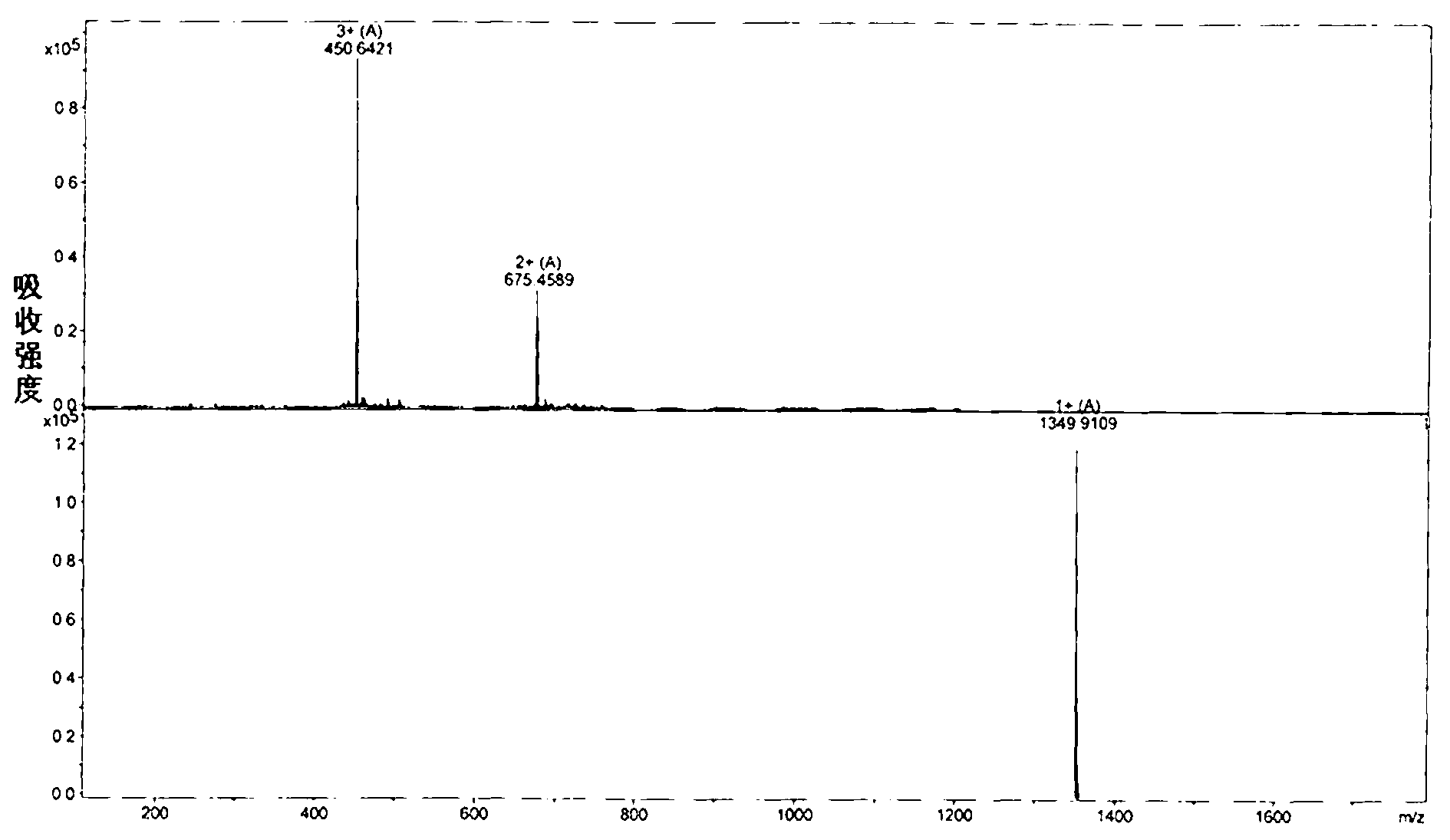
[0117] Embodiment 2: the synthesis of antimicrobial peptide analogue J-AA
[0118] (1) Activation and pretreatment of resin
[0119] With embodiment 1.
[0120] (2) Synthesis of AC-pra-Anoplin-MBHA
[0121] With embodiment 1.
[0122] (3) AC-Nle(N 3 Synthesis of )-Anoplin-MBHA
[0123] a. Coupling of Leu: wash the pretreated resin with DMF 3 times, each time for 2 minutes, after draining, add a mixed solution of DMF containing 20% hexahydropyridine, stir to make it fully react 4 times, each time After the reaction is completed, wash 3 times with DMF, each time for 2 minutes, the indene should be colored, and the resin that has been removed from Fmoc protection is obtained; dissolve 319 mg of Fmoc-protected Leu in DMF, stir to make it fully dissolved, and add HOBT 121.9mg and HBTU 344.2mg, stir to dissolve, then add 300ul DIEA, then mix with the resin deprotected by Fmoc, argon protection, stirring reaction at room temperature for 1h, indene detection colorless, drained,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com