Lithium-air battery cathode bifunctional catalyst and application thereof
A bifunctional catalyst and lithium-air battery technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of large electrode polarization, poor kinetic performance, and low battery charge and discharge efficiency, and achieve long cycle life and low cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: solid-phase synthesis method prepares Ba 0.9 co 0.7 Fe 0.2 Nb 0.1 o 3 (BCFN)
[0031] Weigh 0.1800mol BaCO according to the stoichiometric ratio 3 , 0.0467mol Co 3 o 4 , 0.0200mol Fe 2 o 3 and 0.0100mol Nb 2 o 5 Put it in an agate spheroidal ink tank, mill it in a planetary ball mill for 24 hours, then press it into a tablet, and then put it in a box furnace at 1000 ° C for 24 hours, and then crush it to obtain Ba 0.9 co 0.7 Fe 0.2 Nb 0.1 o 3 .
[0032] Ba 0.9 co 0.7 Fe 0.2 Nb0.1 o 3 The powder needs to be milled by a planetary ball mill for 48 hours and passed through a 100-mesh sieve before making electrode slurry.
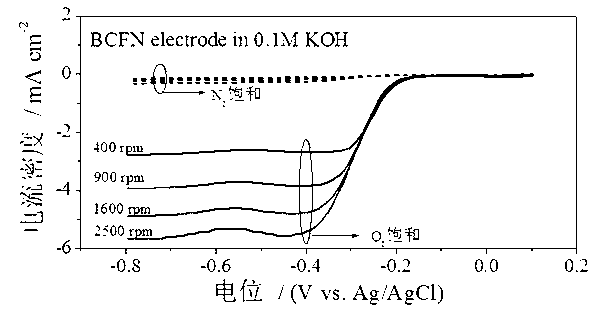
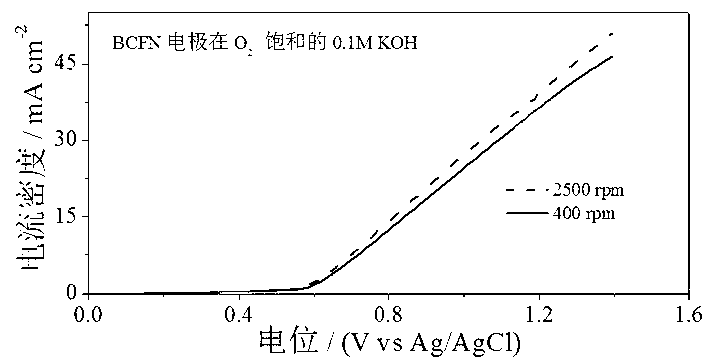
[0033] attached figure 2 for the above Ba 0.9 co 0.7 Fe 0.2 Nb 0.1 o 3 Catalytic activity diagram of the catalyst for the oxygen reduction reaction. It can be seen from the diagram that Ba 0.9 co 0.7 Fe 0.2 Nb 0.1 o 3 The limiting diffusion current density for the oxygen reduction reaction can reach 5.80 mA cm...
Embodiment 2
[0037] Embodiment 2: Sol-gel method prepares Ba 0.5 Sr 0.5 co 0.8 Fe 0.2 o 3 (BSCF)
[0038] Weigh 0.05mol Ba(NO 3 ) 2 , 0.05 mol Sr(NO 3 ) 2 , 0.08 mol Co(NO 3 ) 2 ·6H 2 O and 0.02mol Fe(NO 3 ) 3 9H 2 O was dissolved in deionized water, and 1 mol / L of EDTA-NH 3 Buffer solution, stirred magnetically for 2 hours at room temperature to fully coordinate EDTA and metal ions, then added citric acid with 1.5 times the total molar number of metal ions as a gelling agent, stirred vigorously, and adjusted the pH of the solution to about 6.0 with ammonia water. The above solution was evaporated in a water bath at 80° C. until a sol was formed, and the obtained sol was dried at 200° C. for 24 hours to obtain a dark brown xerogel. The xerogel was calcined at 800 °C for 4 h to obtain black Ba 0.5 Sr 0.5 co 0.8 Fe 0.2 o 3 powder.
[0039] attached Image 6 To utilize the above Ba 0.5 Sr 0.5 co 0.8 Fe 0.2 o 3 Catalytic activity diagram of the catalyst for the oxyge...
Embodiment 3
[0041] Embodiment 3: Glycine nitrate combustion method prepares La 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3
[0042] Weigh 0.06mol La(NO 3 ) 3 9H 2 O, 0.04mol Sr(NO 3 ) 2 , 0.02mol Co(NO 3 ) 3 ·6H 2 O and 0.08mol Fe(NO 3 ) 3 9H 2 O was dissolved in 500ml of deionized water, and then 0.24mol of glycine was added. After the glycine and metal ions fully formed complexes, the mixture was rapidly heated and concentrated until it burned violently to obtain a powdery precursor. After fully grinding the precursor, it was calcined at 800 °C for 4 h to obtain La 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com