Survivin promoter mediated recombinant vector for expressing Gelonin phytotoxin genes and usage thereof
A technology of recombinant vectors and promoters, applied in the field of genetic engineering, can solve the problems of large differences in protein expression and low protein expression, and achieve the effects of inhibiting value growth, broad application prospects, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
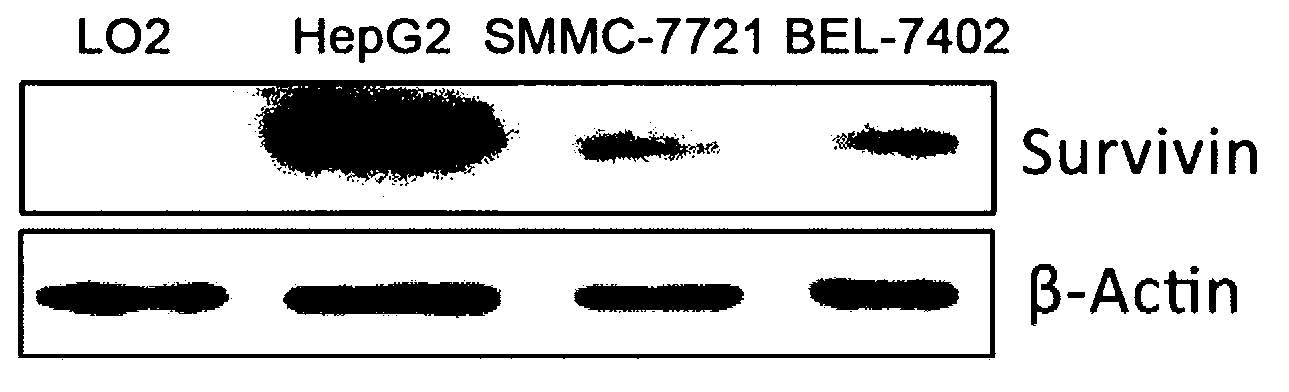
[0073] Example 1 Western blot detection of survivin protein expression
[0074] 1. Extraction of total protein
[0075] 1) Human normal liver cell line LO2, liver cancer cell line HepG2, SMMC-7721, and BEL-7402 cells in good growth state (purchased from the Cell Library of the National Academy of Sciences) were centrifuged and collected in a centrifuge tube after digestion with trypsin. At the same time, the suspended cells in the culture medium were collected together;
[0076] 2) Add RIPA lysate (purchased from Zhongshan Jinqiao Company, add about 400 microliters of RIPA to each bottle of cells) to resuspend the cells by pipetting repeatedly, add 10 microliters of phenylmethylsulfonyl fluoride (PMSF) (100 milliliters), shake well and place on ice;
[0077] 3) Lyse on ice for 30 minutes and mix from time to time;
[0078] 4) After lysis, centrifuge at 12,000 rpm for 5 minutes at 4°C;
[0079] 5) Transfer the centrifuged supernatant to a centrifuge tube for 0.5 minutes an...
Embodiment 2
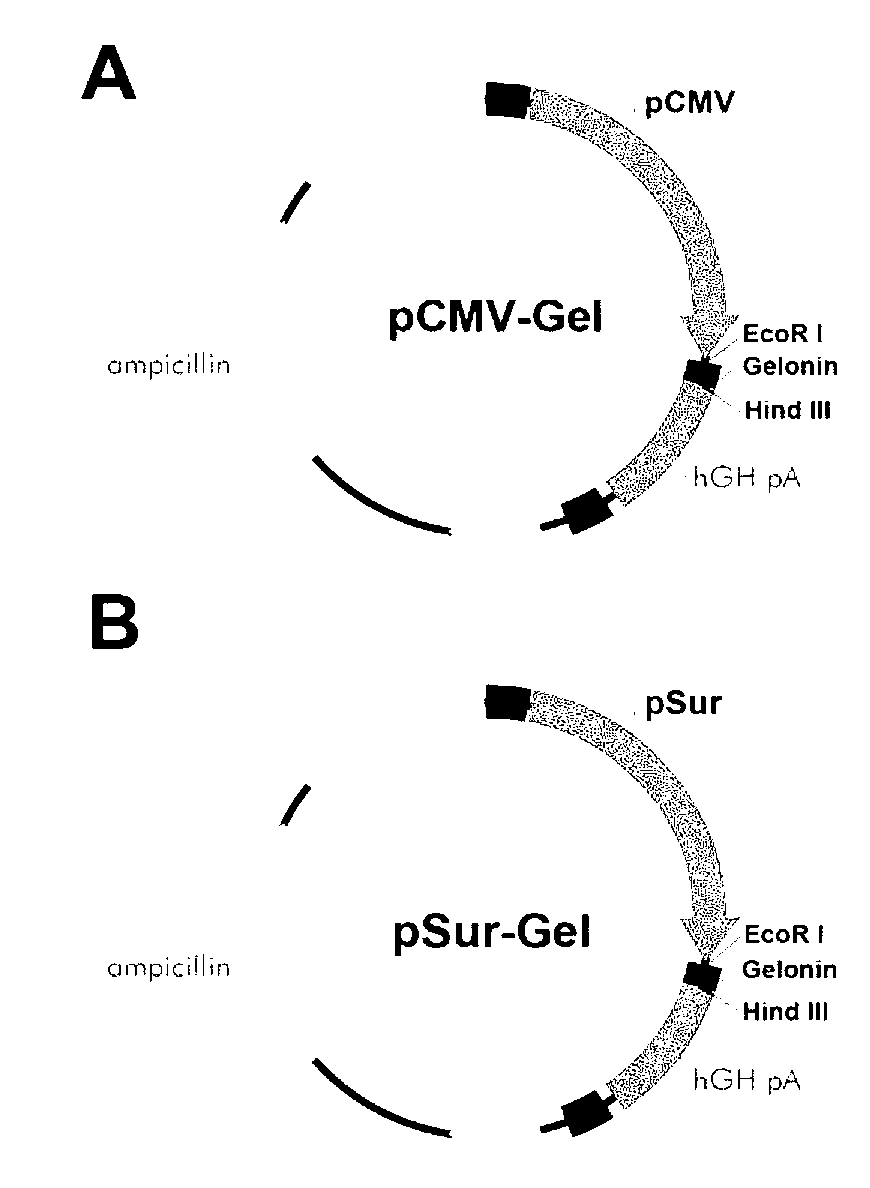
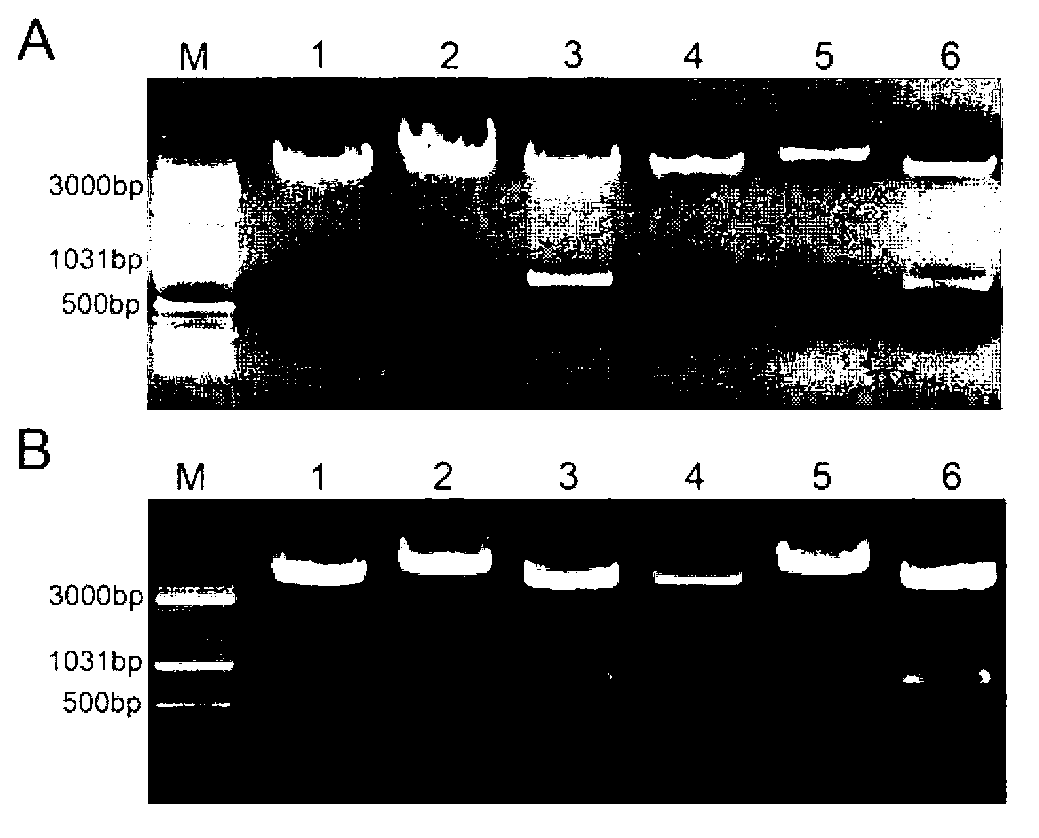
[0097] Example 2 Vector construction
[0098] Since the cytomegalovirus promoter can mediate gene expression in both normal tissue and cancer tissue, we chose the cytomegalovirus promoter. The nucleic acid sequence is shown in SEQ ID No.8. As a control, PAAV was purchased from Cell Biolabs. -MCS expression vector named as pCMV-MCS. Afterwards, the survivin promoter sequence was synthesized by gene, amplified in vitro, and NotI and EcoRI restriction sites were introduced. The amplified survivin promoter and pCMV-MCS vector were subjected to double digestion, and the survivin promoter was connected to the vector, so that the cytomegalovirus promoter was replaced by the survivin promoter, and a pSur-MCS vector was constructed.
[0099] Green fluorescent protein is a reporter molecule that can emit green fluorescence when excited by light in the blue wavelength range, which can be observed under a fluorescent microscope. Due to the characteristic of autofluorescence, it has been...
Embodiment 3
[0122] Example 3 Detection of Survivin promoter activity
[0123] 1. Survivin promoter-mediated green fluorescent egg protein expression activity detection
[0124] Culture LO2 and HepG2 cells, spread six-well plate, about 2.5×10 5 per plate and cultured overnight at 37°C. Wait for the cell confluency to reach 50-60%. By mixing liposomes and plasmids, 2 micrograms of constructed pCMV-GFP and pSur-GFP recombinant plasmids were transiently transferred to each well of cells. Among them, the solid cationic liposome is prepared by our laboratory, and the preparation method of the solid liposome is to use chloroform and ethanol with a volume ratio of 3:1 to mix 1,2-dioleoyl-3- Trimethylaminopropane (DOTAP) and cholesterol (CHOL) were dissolved, and the lipid mixture was dried into a thin layer in a round-bottomed flask. Residual chloroform and ethanol were removed under high vacuum, and then the lipid was washed with 5% Glucose was hydrated, and the lipid was completely dissolve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com