Enterococcus durans and generated bacteriocin for inhibiting listeria monocytogenes
A technology of durable enterococcus and listeria, applied in the field of microorganisms, can solve the problems of difficult promotion and high equipment, and achieve the effect of simple method, high efficiency and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0040] Screening and Identification of Example 1 Durable Enterococcus (Enterococcus durans) NJ Strain
[0041] MRS medium contains: 9.0g / L peptone, 6.0g / L beef extract powder, 4.5g / L yeast extract powder, 18.0g / L glucose, 1.0mL / L Tween 80, 2.0g / L dipotassium hydrogen phosphate, 5.0g / L sodium acetate, 2.0g / L triammonium citrate, 0.2g / L magnesium sulfate, 0.06g / L manganese sulfate, the pH value is 6.5.
[0042] After 10-fold gradient dilution of traditional fermented dairy products from Inner Mongolia, spread them on the MRS medium, isolate single colonies, control the number of single colonies in each plate to be between 100-400, and culture overnight in a 37°C incubator. Then pick up the culture medium covered with single colonies in the plate, put it horizontally on the agar plate inoculated with 0.5% fresh Listeria (L.monocytogenes CMCC54004, purchased from China Institute for the Control of Pharmaceutical and Biological Products, the same below), and continue to cultivate ...
Embodiment 2
[0044] Implementation example 2 Preparation of bacteriocin produced by Enterococcus durans NJ strain
[0045] (1) Preparation of fermentation broth of Enterococcus durans NJ strain
[0046] Seed medium formula: glucose 20.0g / L, peptone 10.0g / L, beef extract powder 10.0g / L, yeast extract powder 5.0g / L, Tween-801.0mL / L, dipotassium hydrogen phosphate 2.0g / L, Sodium acetate 5.0g / L, diamine hydrogen citrate 2.0g / L, magnesium sulfate 0.2g / L, manganese sulfate 0.05g / L, pH value 6.2, solvent is water.
[0047] Fermentation medium 1 formula: glucose 18.0g / L, peptone 9.0g / L, beef extract powder 6.0g / L, yeast extract powder 4.5g / L, Tween-801.0mL / L, dipotassium hydrogen phosphate 2.0g / L , sodium acetate 5.0g / L, triammonium citrate 2.0g / L, magnesium sulfate 0.2g / L, manganese sulfate 0.06g / L, pH value 6.5, solvent is water.
[0048] Pre-treatment of fermentation medium 1: Rinse AMBERLITE XAD16 resin (purchased from Sigma) with pH 2, 70% by volume isopropanol aqueous solution, and then us...
Embodiment 3
[0071] Example 3 Properties of Bacteriocins Produced by Enterococcus durans NJ Strain
[0072] After the fermentation broth 1 in Example 2 was centrifuged, the obtained fermentation broth supernatant was subjected to the following property determination.
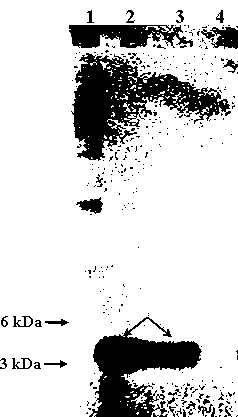
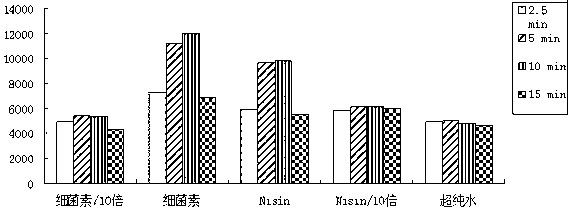
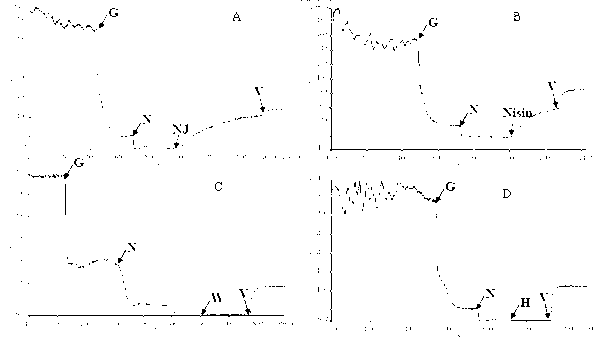
[0073] (1) pH stability: the pH value of the supernatant of Enterococcus durans NJ strain fermentation broth was adjusted to pH 2, 3, 4, 5, 6, 7, 8, 9, 10 and 11 with hydrochloric acid, and then Incubate at 37°C for 2 hours. Using the double dilution method, drop 5ul of the supernatant of the fermentation broth on the BHI solid medium inoculated with 0.5% fresh Listeria, cultivate overnight, and observe the maximum dilution that can inhibit the growth of Listeria. It was found that under various pH conditions, the maximum dilution of the supernatant of the fermentation broth that could inhibit the growth of Listeria was 8 times. The results indicated that the bacteriocin produced by the NJ strain of Enterococcus durans had...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com