6-(beta-alkylnaphthy)mercaptopurine compound, and synthesis method and application thereof
A technology of mercaptopurine and synthetic method, which is applied in organic chemistry, drug combination, antineoplastic drugs, etc., to achieve remarkable effect, simple synthesis steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
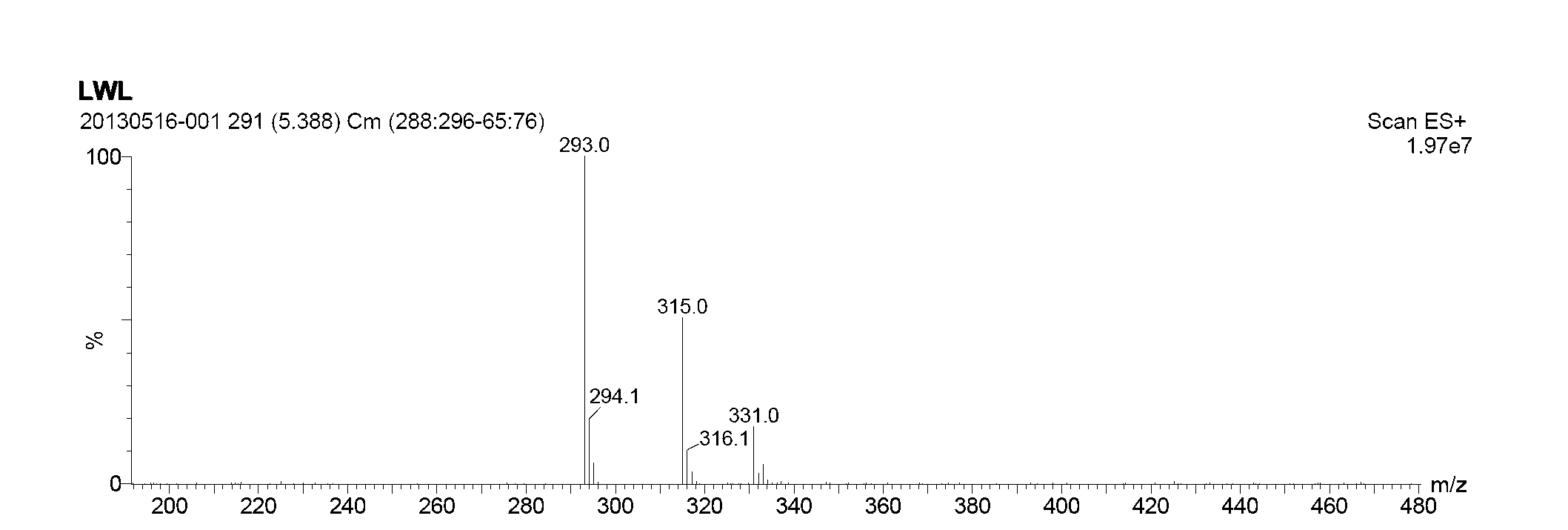
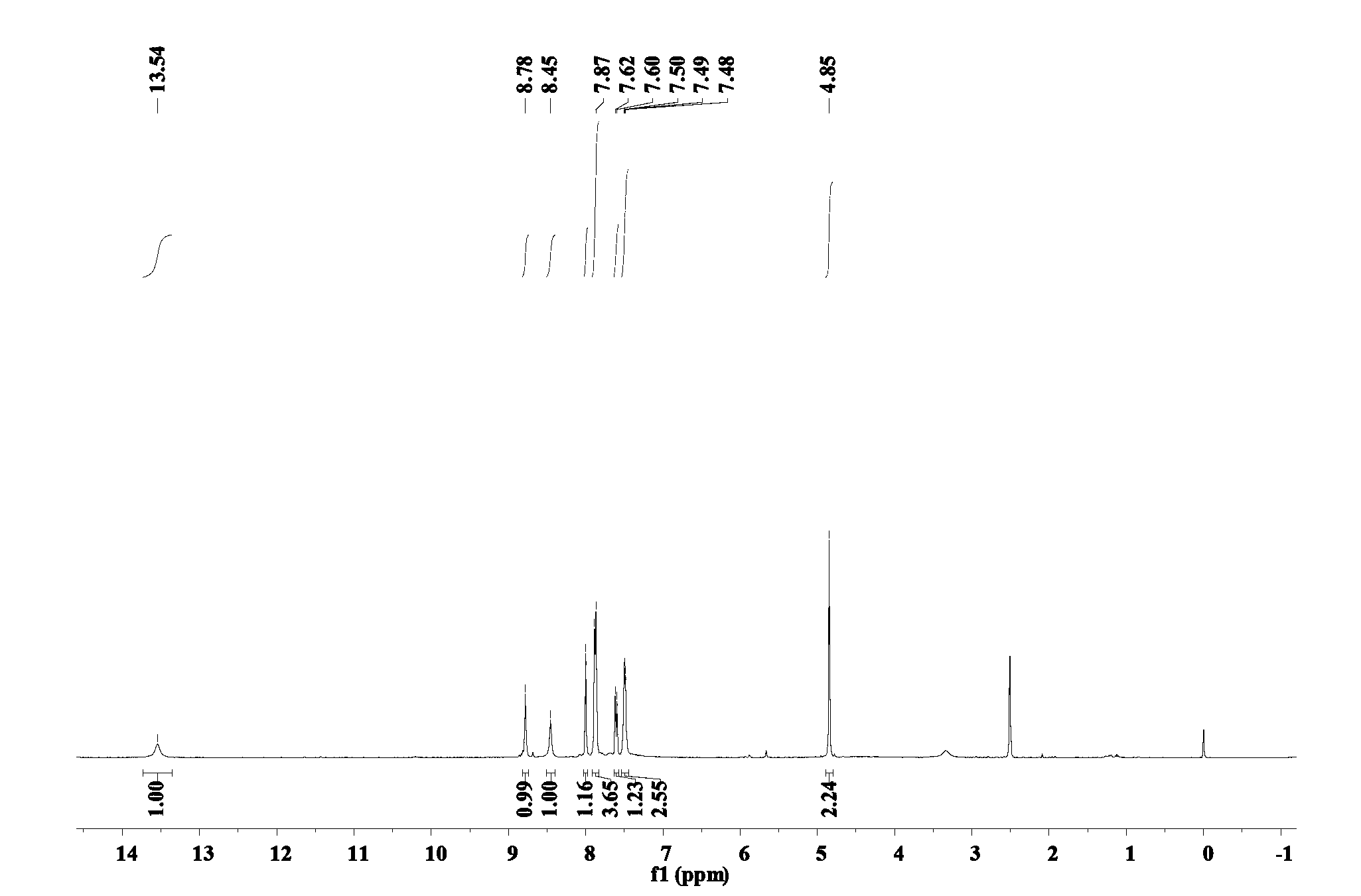
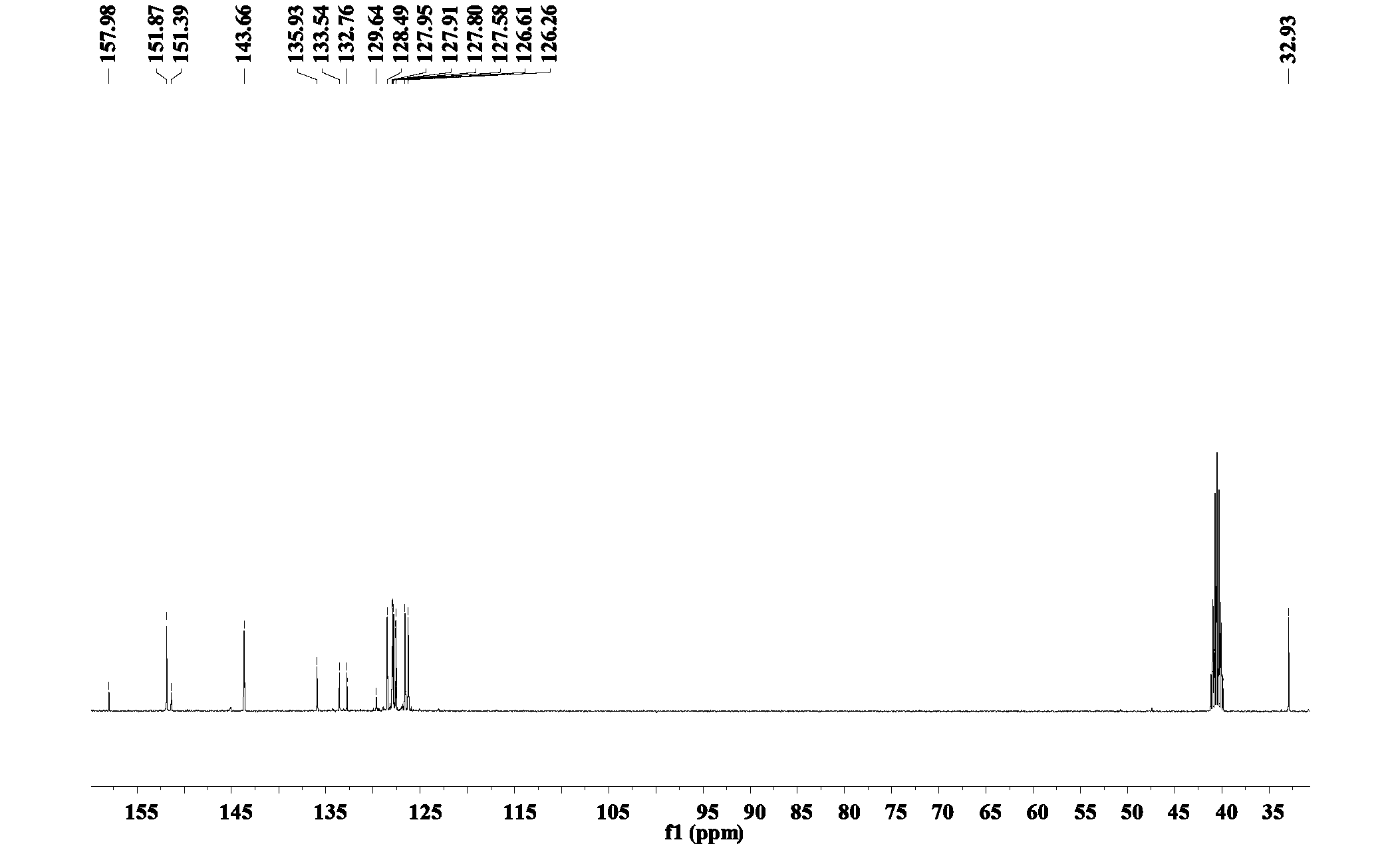
Image
Examples
Embodiment 1
[0024] 1. Synthesis of 6-(β-methylnaphthalene) mercaptopurine compound
[0025] (1) In an inert gas atmosphere, anhydrous and oxygen-free reactor, add the solvent N,N-dimethylacetamide dissolved in 6-mercaptopurine, the mass fraction of 6-mercaptopurine is 0.34 parts, N , the mass fraction of N-dimethylacetamide is 10 parts;
[0026] (2) Add 0.7 parts of potassium carbonate and 0.53 parts of 2-bromomethylnaphthalene to the above reactor in turn, and react at room temperature for 4.5 hours;
[0027] (3) After the reaction is completed, add 10 parts of deionized water to the above system, and then add acetic acid for neutralization to form a precipitate;
[0028] (4) Remove the supernatant of the above mixture, and then wash the precipitate with deionized water and ether respectively to obtain a crude product of white solid, which is recrystallized with the solvent toluene to obtain the final product 6-(β- Methylnaphthalene) mercaptopurine compound, the productive rate is 66.5...
Embodiment 2
[0037] 1. Synthesis of 6-(β-ethylnaphthalene) mercaptopurine compound
[0038] (1) In an inert gas atmosphere, anhydrous and oxygen-free reactor, add the solvent N,N-dimethylacetamide dissolved in 6-mercaptopurine, the mass fraction of 6-mercaptopurine is 1, the solvent N , the mass fraction of N-dimethylacetamide is 20 parts;
[0039] (2) Add 1 part of potassium carbonate and 1.2 parts of 2-bromoethylnaphthalene to the above reactor in turn, and react at room temperature for 7 hours;
[0040] (3) After the reaction is completed, add 25 parts of deionized water to the above system, and then add acetic acid for neutralization to form a precipitate;
[0041] (4) Remove the supernatant of the above mixture, and then wash the precipitate with deionized water and ether respectively to obtain a crude product of a white solid, which is recrystallized with the solvent xylene to obtain the final product 6-(β -ethylnaphthalene) mercaptopurine compound, the productive rate is 72.18%. ...
Embodiment 3
[0045] 1. Synthesis of 6-(β-methylnaphthalene) mercaptopurine compound
[0046] (1) In an inert gas atmosphere, anhydrous and oxygen-free reactor, add the solvent N,N-dimethylacetamide dissolved in 6-mercaptopurine, the mass fraction of 6-mercaptopurine is 0.6 parts, N, The mass fraction of N-dimethylacetamide is 10 parts;
[0047](2) Add 0.7 parts of potassium carbonate and 0.8 parts of 2-bromomethylnaphthalene to the above reactor in turn, and react at room temperature for 4.5 hours;
[0048] (3) After the reaction is completed, add 17 parts of deionized water to the above system, and then add acetic acid for neutralization to form a precipitate;
[0049] (4) Remove the supernatant of the above mixture, and then wash the precipitate with deionized water and ether respectively to obtain a crude product of white solid, which is recrystallized with the solvent toluene to obtain the final product 6-(β- Methylnaphthalene) mercaptopurine compound, the productive rate is 69.64%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com