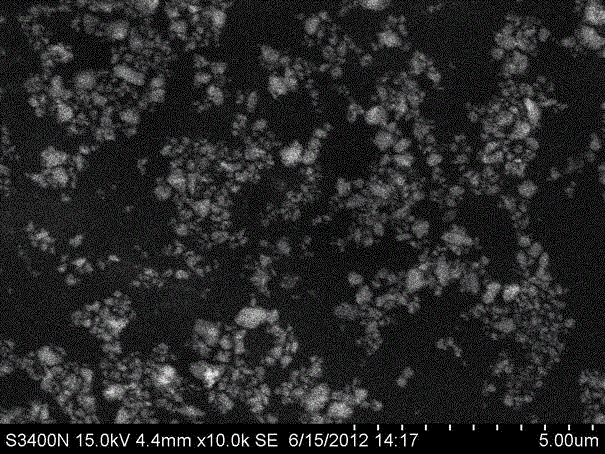
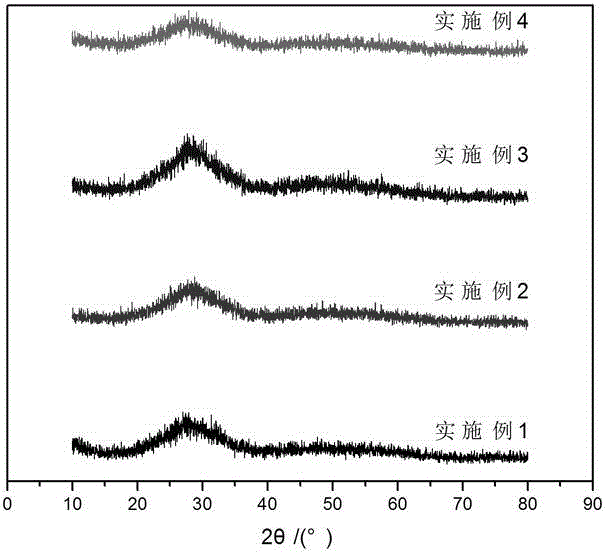
Lead-free superfine glass powder and synthetic method thereof
A technology of ultra-fine glass powder and synthesis method, which is applied in the field of electronic materials, can solve the problems of difficult uniformity of melt, high energy consumption of glass, and difficult refinement of powder, etc., and achieve large linear expansion coefficient and softening temperature range, chemical composition uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Select the glass oxide component precursor, bismuth nitrate, tetraethyl orthosilicate, boric acid, zinc acetate or zinc nitrate, aluminum isopropoxide or aluminum nitrate as the Bi 2 o 3 , SiO 2 , B 2 o 3 , ZnO, Al 2 o 3 Precursors, using deionized water and absolute ethanol, citric acid, and nitric acid as solvents, complexing agents, and catalysts;
[0033] (2) According to the glass composition oxide Bi 2 o 3, SiO 2 , B 2 o 3 , ZnO, Al 2 o 3 The weight percentages of 55%, 25%, 10%, 7%, and 3% are converted to the weight of the precursor.
[0034] (3) Weigh the precursor, prepare ethyl orthosilicate, absolute ethanol, A mixed solution of deionized water was placed in a constant temperature water bath at 55°C, nitric acid was added dropwise as a catalyst, the pH was controlled to be 2.5, and an electric stirrer was used to fully stir it to make it hydrolyze for 35 minutes.
[0035] (4) According to the weight ratio of bismuth nitrate: dilute nitric ac...
Embodiment 2
[0041] (1) Select the glass oxide component precursor, bismuth nitrate, tetraethyl orthosilicate, boric acid, zinc acetate or zinc nitrate, aluminum isopropoxide or aluminum nitrate as the Bi 2 o 3 , SiO 2 , B 2 o 3 , ZnO, Al 2 o 3 Precursors, using deionized water and absolute ethanol, citric acid, and nitric acid as solvents, complexing agents, and catalysts;
[0042] (2) According to the glass composition oxide Bi 2 o 3 , SiO 2 , B 2 o 3 , ZnO, Al 2 o 3 The weight percentages of 50%, 30%, 10%, 8%, and 2% are converted to the weight of the precursor.
[0043] (3) Weigh the precursor, prepare ethyl orthosilicate, absolute ethanol, The mixed solution of deionized water is placed in a constant temperature water bath at 50°C, nitric acid is added dropwise as a catalyst, the pH is controlled to be 3, and an electric stirrer is used to fully stir it to make it hydrolyze for 30 minutes.
[0044] (4) According to the weight ratio of bismuth nitrate:dilute nitric acid=1...
Embodiment 3
[0048] (1) Select the glass oxide component precursor, with bismuth nitrate, ethyl orthosilicate, boric acid, zinc acetate or zinc nitrate, aluminum isopropoxide or aluminum nitrate as Bi respectively 2 o 3 , SiO 2 , B 2 o 3 , ZnO, Al 2 o 3 Precursors, using deionized water and absolute ethanol, citric acid, and nitric acid as solvents, complexing agents, and catalysts;
[0049] (2) According to the glass composition oxide Bi 2 o 3 , SiO 2 , B 2 o 3 , ZnO, Al 2 o 3 The weight percentages of 45%, 25%, 25%, 4%, and 1% are converted to the weight of the precursor.
[0050] (3) Weigh the precursor, prepare ethyl orthosilicate, absolute ethanol, The mixed solution of deionized water is placed in a constant temperature water bath at 55°C, nitric acid is added dropwise as a catalyst, the pH is controlled to be 3.2, and an electric stirrer is used to fully stir it to make it hydrolyze for 35 minutes.
[0051] (4) According to the weight ratio of bismuth nitrate:dilute ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com