3,3'-(3,4-dichlorobenzylidene)-bis-4-hydroxycoumarin and application thereof in preparation of medicine for resisting multi-drug resistant bacteria
A technology of dichlorobenzylidene and hydroxycoumarin, which is applied in the direction of antibacterial drugs, active ingredients of heterocyclic compounds, organic chemistry, etc., can solve the toxicity of coumarin compounds, low success rate of antibacterial effect, and can not be applied Body and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
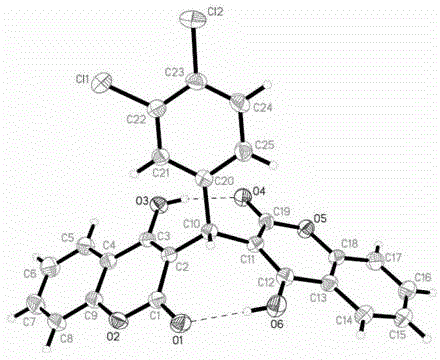
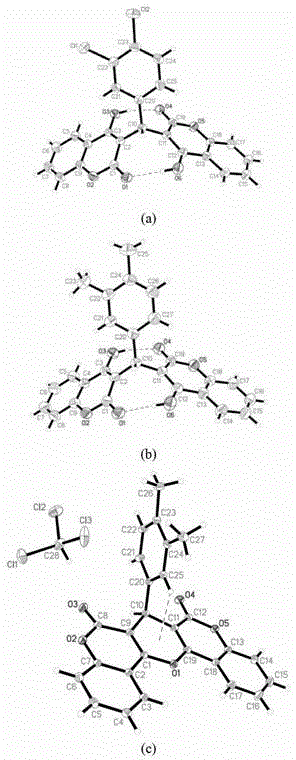
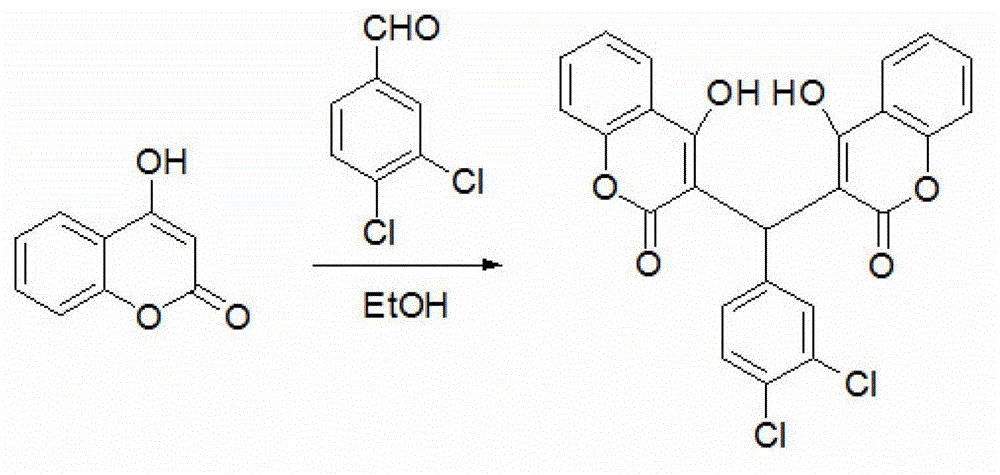
[0028] Example 1: Chemical synthesis, crystal incubation and structure identification of compound DCH. In this embodiment, the specific test procedure is as follows.
[0029] In the first step, 10g of 4-hydroxycoumarin and 100mL of absolute ethanol were added to a 250mL three-necked flask, and heated until the 4-hydroxycoumarin was dissolved.
[0030] In the second step, 5.2g of 3,4-dichlorobenzaldehyde is added and heated to reflux for 3-4 hours.
[0031] In the third step, white solid particles are precipitated, and heating is continued for about 1 hour. After the reaction is completed, it is naturally cooled and filtered with suction, and then recrystallized with 95% ethanol to finally obtain pure white granular crystals.
[0032] The fourth step is to cultivate single crystals by the solution interface diffusion method: dissolve a small amount of compound DCH in dichloromethane solution, and carefully spread the acetone solution on it. The volume ratio is dichloromethane:acetone=3...
Embodiment 2
[0037] Example 2: Determination of the minimum inhibitory concentration of compound DCH
[0038] The first step, the method of gradient dilution of DCH and antibacterial drugs: Weigh 2 mg of compound DCH or control antibacterial drugs into 1 ml of diluent and mix well. The concentration of the original drug solution is 2 mg / ml. After the original solution is diluted, it is sterilized by filtration, and a small amount is used for equipment. The stock solution can be stored for 3 months below -20°C, but it can only be stored for one week at 4°C. 512μl stock solution was added to 488μl M-H broth medium, and the highest concentration of antibacterial drug after mixing was 2048μg / ml. Suction 100μl of the drug with the highest concentration and add it to the No. 1 well of each row. After mixing the No. 1 well, aspirate 100μl, add it to the No. 2, and dilute to No. 10 in sequence. Aspirate 100μl and discard, so that the gradient concentration of the drug is 1024, 512, 256, 128, 64, 32...
Embodiment 3
[0045] Example 3: Determination of the compound DCH bactericidal curve.
[0046] In the first step, take 8 sterile Erlenmeyer flasks and label them as "Growth Control Tube and Compound DCH". Add 6ml nutrient broth to each bottle.
[0047] In the second step, the quantitative compound DCH was added into each conical flask, the content of which was 32 μg / ml concentration, and the growth control group was added with the same volume of deionized water.
[0048] The third step is to add 60μl of the tested bacterial solution Staphylococcus aureus (S.aureas, ATCC29213) and methicillin-resistant Staphylococcus aureus (MRSA, XJ75302) with a concentration of 0.5 Maid's turbidity standard into each conical flask , USA300 (LAC) and Vancomycin-resistant Staphylococcus aureus (Mu50ATCC700699), the final concentration of each tube of bacteria solution is about 10 6 CFU / ml.
[0049] In the fourth step, immediately vortex the conical flask for 15 seconds after adding bacteria, and after 10 times of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com