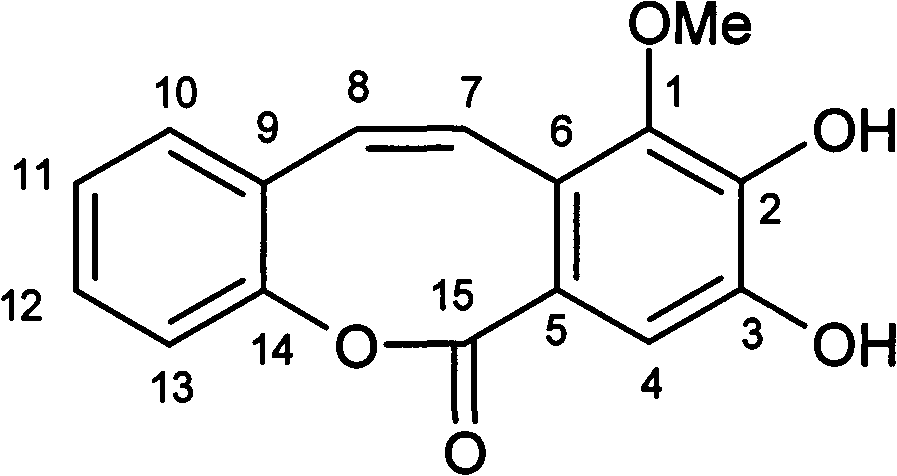
A novel compound sciryagarol II having antineoplastic and antibiosis activities and a preparation method thereof
A compound and anti-tumor technology, applied in the field of medicine, can solve the problems of unreported cis-stilbene compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] The preparation method of embodiment 1 trigonol II
[0013] Take 20Kg of dried tubers of Scirpus yagara purchased from Zhejiang, crush them, heat and reflux them with 80% ethanol aqueous solution for extraction three times, combine the three extracts, concentrate under reduced pressure until there is no alcohol smell, dilute the concentrate with water to form a suspension, and separate With petroleum ether, ethyl acetate extraction. The ethyl acetate extracts were combined and concentrated to obtain 60.9 g of extract, and the extract was subjected to silica gel column (200-300 mesh) chromatography, with petroleum ether: ethyl acetate gradient (100:0, 100:2, 100:4, ... ..., 100:20) elution, GF254 silica gel thin-layer chromatography, ultraviolet inspection, collect the fraction containing cinnamonol II, combine and concentrate and then go through silica gel column chromatography, and use petroleum ether-ethyl acetate gradient (100:5 , 100:7, 100:9,..., 100:15) elution, ...
Embodiment 2
[0014] Example 2 Inhibitory effect of tricerol II on human cervical cancer Hela cells, human liver cancer SMMC-7721 cells and human gastric cancer MGC-803 cells
[0015] Hela cells (3×10 4 cells / mL), SMMC-7721 and MGC-803 cells (5×10 4 cells / mL), were inoculated on a 96-well plate at 100 uL per well, and 4 duplicate wells were set up. Hela cells were cultured for 10 hours, and the culture medium was discarded. After SMMC-7721 and MGC-803 cells were cultured for 20 hours, the culture medium was discarded, and then the medium was discarded. Add 100uL / well of each drug at different concentrations, the highest concentration of each drug is 100uM / L, 10 times the dose interval, set five doses, and each dose has four replicate wells. Add 100uL DMSO with a final concentration of 0.1% to the negative control group, add 100uL monoculture to the blank group, culture Hela cells for 36 hours, and culture SMMC-7721 and MGC-803 for 24 hours, add 20 μL of MTT solution with a concentration of...
Embodiment 3
[0016] The inhibitory effect of embodiment 3 trichosanol II on Staphylococcus aureus, Escherichia coli and Candida albicans
[0017] Take a few standard strains: Staphylococcus aureus (ATCC25923) and Escherichia coli (441490) strains, inoculate them in nutrient broth medium respectively, and incubate at 37°C for 18h. Get each bacterial strain nutrient broth culture culture of 18h cultivation, make 10 with nutrient broth -3 diluted for experiments. Take a little strain of Candida albicans, inoculate it in modified Martin's medium, and culture it at 35°C for 24h. Take the culture of Candida albicans cultivated for 24h, and use the modified Martin's medium 10 -3diluted for experiments. Prepare the sample with the double dilution method (test tube method), that is, the initial concentration of the test drug cinnamonol II is 10mg / ml, take 0.1ml and add 5.9ml nutrient broth to dilute and filter for later use, take 8 sterilized test tubes, the first Add 1ml of the test drug dilut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com