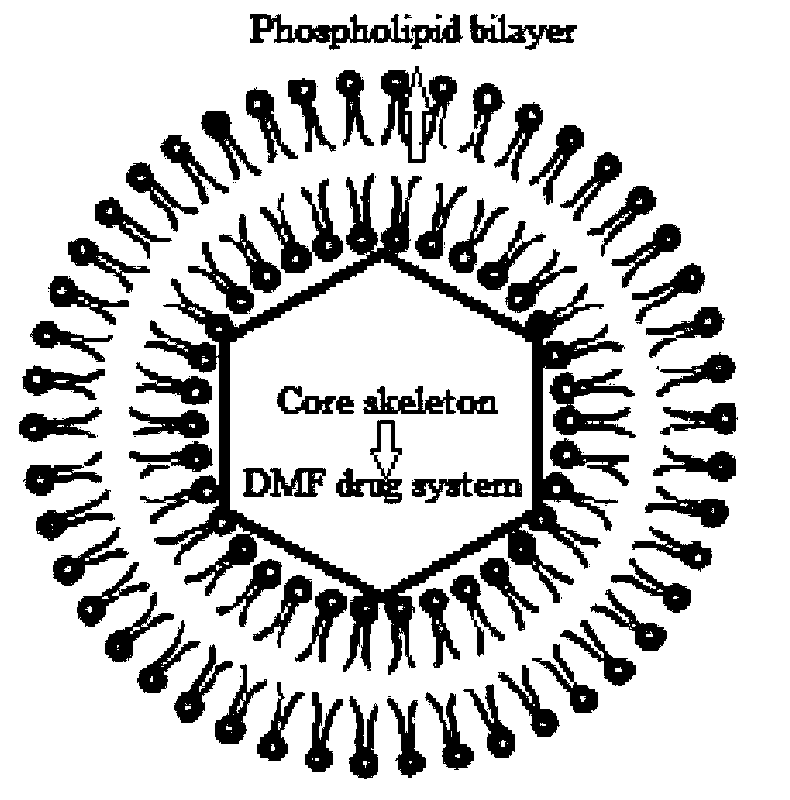
Dextran-MLDH-fluorouracil super-molecular skeleton magnetic liposome
A magnetic liposome, fluorouracil technology, applied in the directions of liposome delivery, organic active ingredients, medical preparations of non-active ingredients, etc., can solve the problem of easy torn lipid capsule wall, drug leakage, lack of internal bonding Spatial distribution and other issues to achieve significant magnetic targeting specificity and stable plasma concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
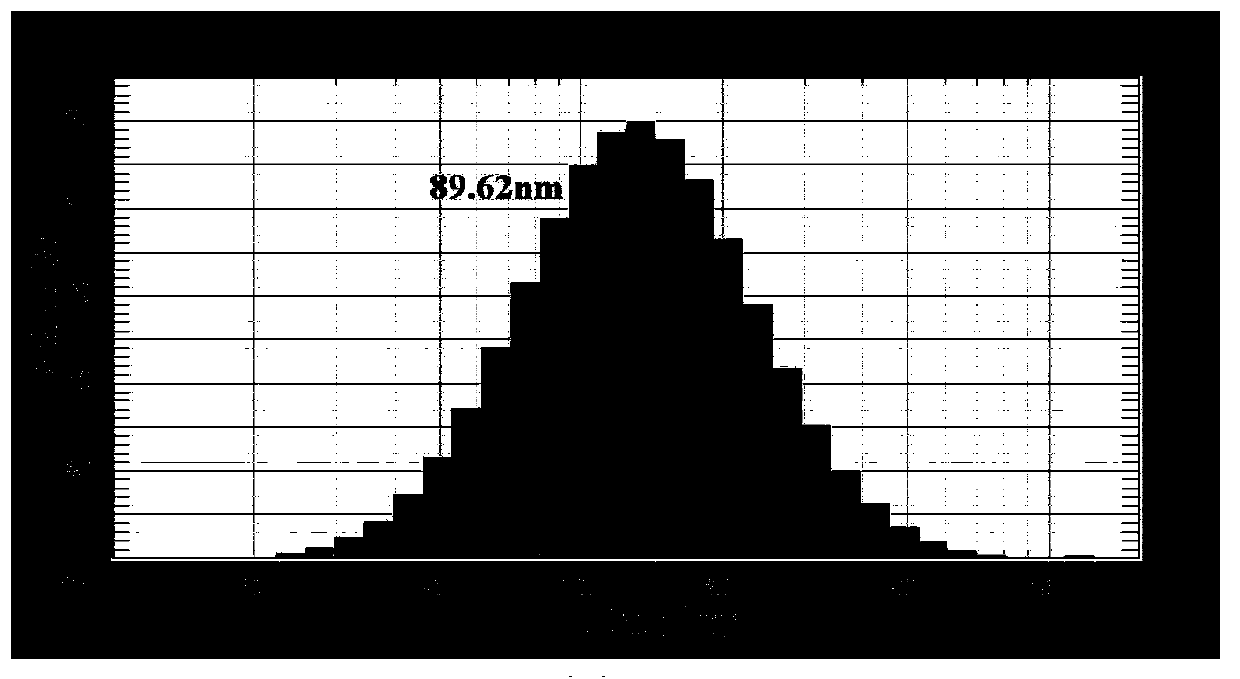
[0028] Example 1 Synthesis of DMFL liposomes, particle morphology and particle size distribution of characterization products
[0029] prescription:
[0030] DMF
6mg
30mg
10mg
15mL
double distilled water
30mL
[0031] Preparation Process:
[0032] (1) Synthesis of DMF drug carrier: Weigh 39.76g of ferrous chloride and 27.03g of ferric chloride and put them in a sulfuric acid paper bag for later use; weigh 84g of dextran (DET) and dissolve it in 200mL of water; measure 69mL of 0.145mol L -1 The Fu solution is set aside. Assemble the nitrogen protection reaction system with a pH meter, stirring magnet, precipitant infusion drive system and 1000mL three-necked bottle, add 300mL double distilled water, and 2 1. Add two kinds of iron salts under the condition of magnetic stirring, add the solution after dissolving, stir and mix well, keep the temperature at 45°C and N 2 Under pr...
Embodiment 2
[0039] Example 2 Synthesis of DMFL according to the following prescription, detection of liquid phase pH value and in vitro magnetic targeting
[0040] DMF
6mg
42mg
14mg
l5mL
double distilled water
20mL
[0041] Preparation process: The process flow for the synthesis of DMF and DMFL is the same as in Example 1. The raw materials were prepared according to the prescription list, the lipid film preparation temperature was 50°C, and emulsified for 30 minutes; FU original drug liposomes were prepared under the same prescription and process conditions, and were used as comparison samples for DMFL magnetic targeting verification.
[0042] Detection of liquid phase pH value and magnetic targeting in vitro: the pH value of DMFL was measured with a PHSJ-4A acidity meter. Take the same amount of DMF liposomes and FU liposomes, and pack them in different ampoules, use Φ17×3mm NdFeB perma...
Embodiment 3
[0043] Example 3 Synthesize DMFL according to the following prescription, detect in vitro release performance and cancer cell proliferation inhibition
[0044] DMF
6mg
30mg
cholesterol
10mg
l5mL
double distilled water
3OmL
[0045] Preparation process: The synthesis process of DMF and DMFL is the same as in Example 1. The dosage of each component is shown in the prescription table. The lipid film preparation temperature is 45° C., emulsification for 30 minutes. Four batches of parallel samples of DMFL were synthesized under the same prescription conditions for the tests of encapsulation efficiency and pH; liposomes of FU original drug were prepared under the same conditions for in vitro release performance and tumor cell proliferation inhibitory activity tests as reference substances.
[0046] Determination of the drug encapsulation efficiency of liposomes: determined by dialysis. Precise...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com