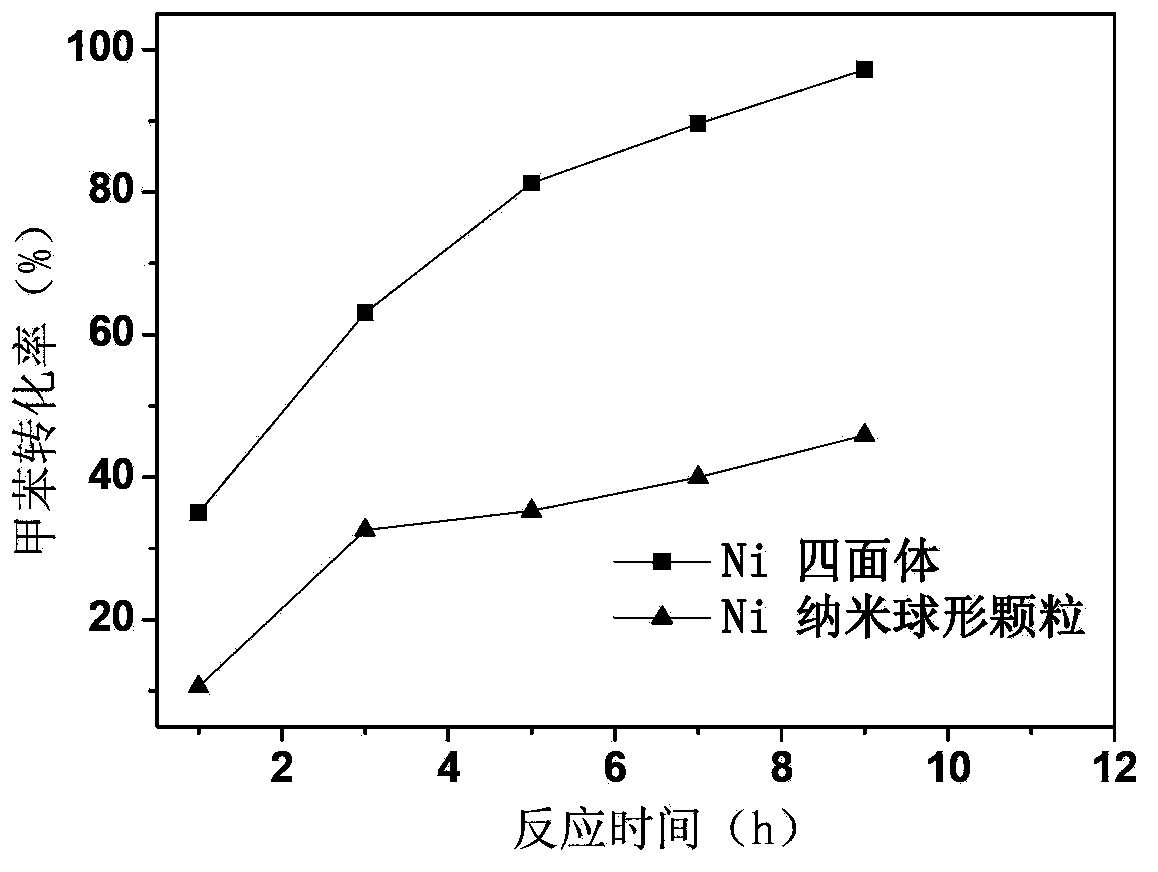
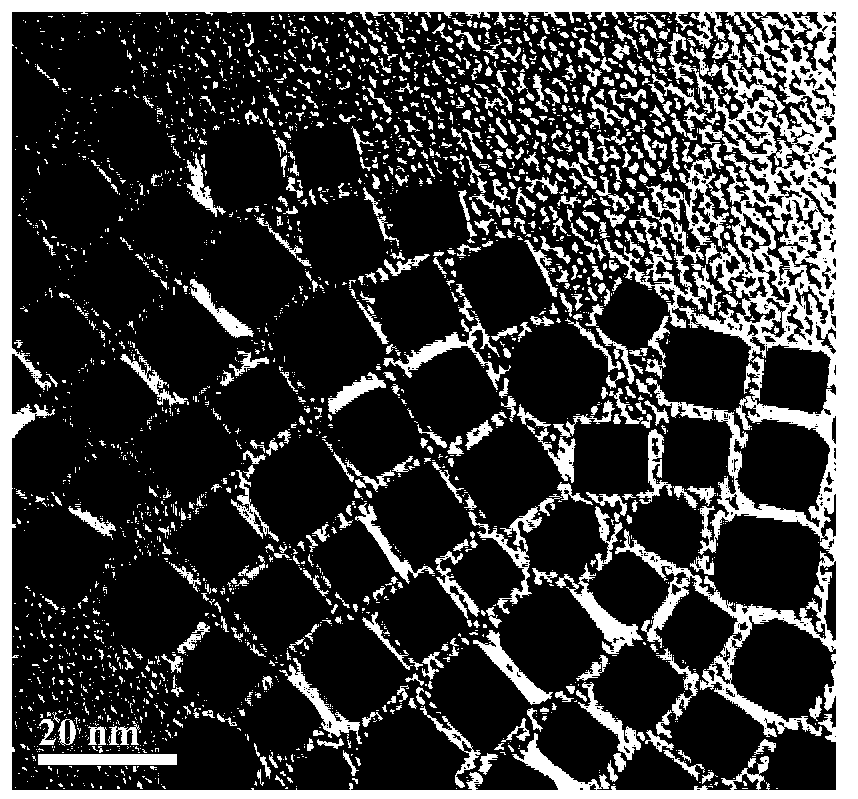
Nanometer nickel-based polyhedron catalyst used for aromatic ring hydrogenation, preparation method thereof and application thereof
A catalyst and polyhedron technology, applied in the field of nano-nickel-based polyhedron catalysts, to achieve the effects of stable structure, mild reaction conditions, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Dissolve 0.003mol of nickel chloride and 0.036mol of sodium citrate [molar ratio 1:12] in 70mL of ethylene glycol, and stir to dissolve under heating at 45°C to obtain a uniform solution 1.
[0045] Then 0.006 mol of sodium hydroxide and 0.003 mol of benzoic acid [the molar ratio of the two is 2:1] were dissolved together in 15 mL of ethylene glycol, and the obtained solution was added dropwise to the above solution 1 to obtain solution 2.
[0046] Then, 0.018 mmol of sodium formate was weighed and dissolved in 15 mL of ethylene glycol to obtain solution 3.
[0047]Put solution 2 and solution 3 in a 90°C vacuum oven and dry for 30 minutes. After taking them out, quickly mix them (dried solution 2 and solution 3) in a 250mL three-necked bottle. Under the temperature of 220 ℃ of oil baths and accompanied by condensing and reflux, stirring and reacting for 18 hours, the product obtained was washed and separated with ethanol, and the reaction product was vacuum-dried at 75 ...
Embodiment 2
[0052] Dissolve 0.003mol of nickel nitrate and 0.03mol of potassium citrate [the molar ratio of the two is 1:10] in 70mL of ethylene glycol, stir and dissolve under heating at 45°C to obtain a uniform solution 1.
[0053] Then 0.027mol potassium hydroxide and 0.015mol benzoic acid [the molar ratio of the two is 9:5] were dissolved in 15mL of ethylene glycol, and the obtained solution was added dropwise to the above solution 1 to obtain solution 2.
[0054] Then, 0.036 mol of potassium formate was weighed and dissolved in 18 mL of ethylene glycol to obtain solution 3.
[0055] Dry solution 2 and solution 3 in a vacuum oven at 75°C for 50 minutes. After taking them out, quickly mix the two (dried solution 2 and solution 3) evenly in a 250mL three-neck bottle. Under a nitrogen atmosphere, condense and reflux reaction at 200°C oil bath temperature for 20 hours, wash and separate the obtained product with ethanol, and vacuum dry the reaction product at 90°C for 10 hours to obtain a...
Embodiment 3
[0058] Dissolve 0.003mol of nickel nitrate and 0.045mol of sodium citrate [the molar ratio of the two is 1:15] in 70mL of ethylene glycol, stir and dissolve under heating at 45°C to obtain a uniform solution 1.
[0059] Then 0.009 mol of sodium hydroxide and 0.006 mol of benzoic acid [the molar ratio of the two is 3:2] were dissolved together in 15 mL of ethylene glycol, and the obtained solution was added dropwise to the above solution 1 to obtain solution 2.
[0060] Then, 0.024 mol of sodium formate was weighed and dissolved in 15 mL of ethylene glycol to obtain solution 3.
[0061] Put solution 2 and solution 3 at 95°C for 35 minutes in vacuum, and after taking them out, quickly mix them (dried solution 2 and solution 3) evenly in a 250mL three-neck bottle. Under a nitrogen atmosphere, condense and reflux at a temperature of 215°C for 10 hours, wash and separate the obtained product with ethanol, and dry the reaction product in vacuum at 80°C for 16 hours to obtain a nanom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com