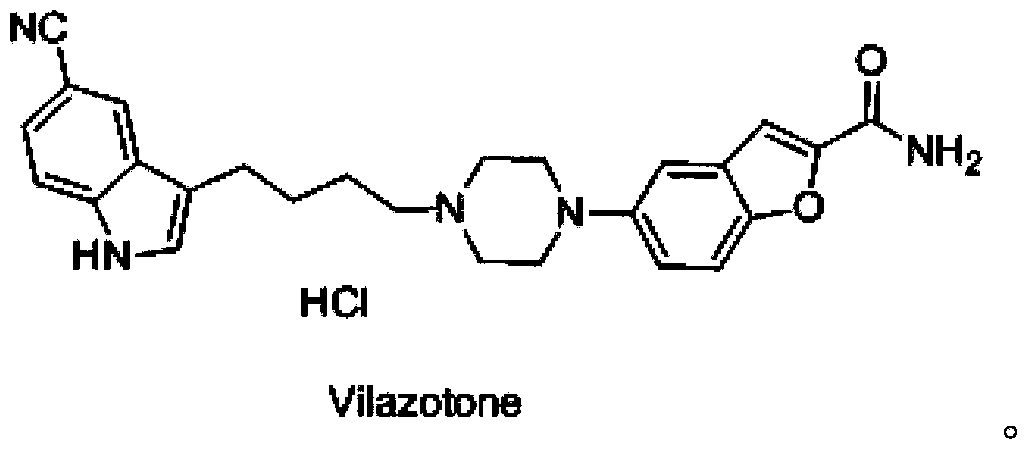
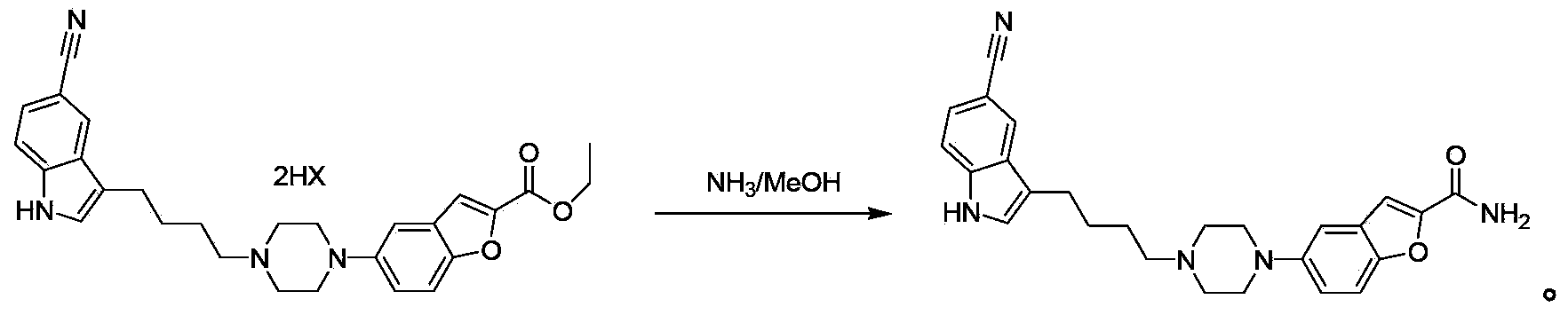
Synthesis method of vilazodone intermediate and salt thereof
A synthesis method and step method technology are applied in the field of synthesis of pharmaceutical intermediates, which can solve the problems of operator health hazards, unfavorable industrialized production, long reaction time and the like, and achieve less environmental pollution, simple and easy post-reaction treatment, and reaction steps. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
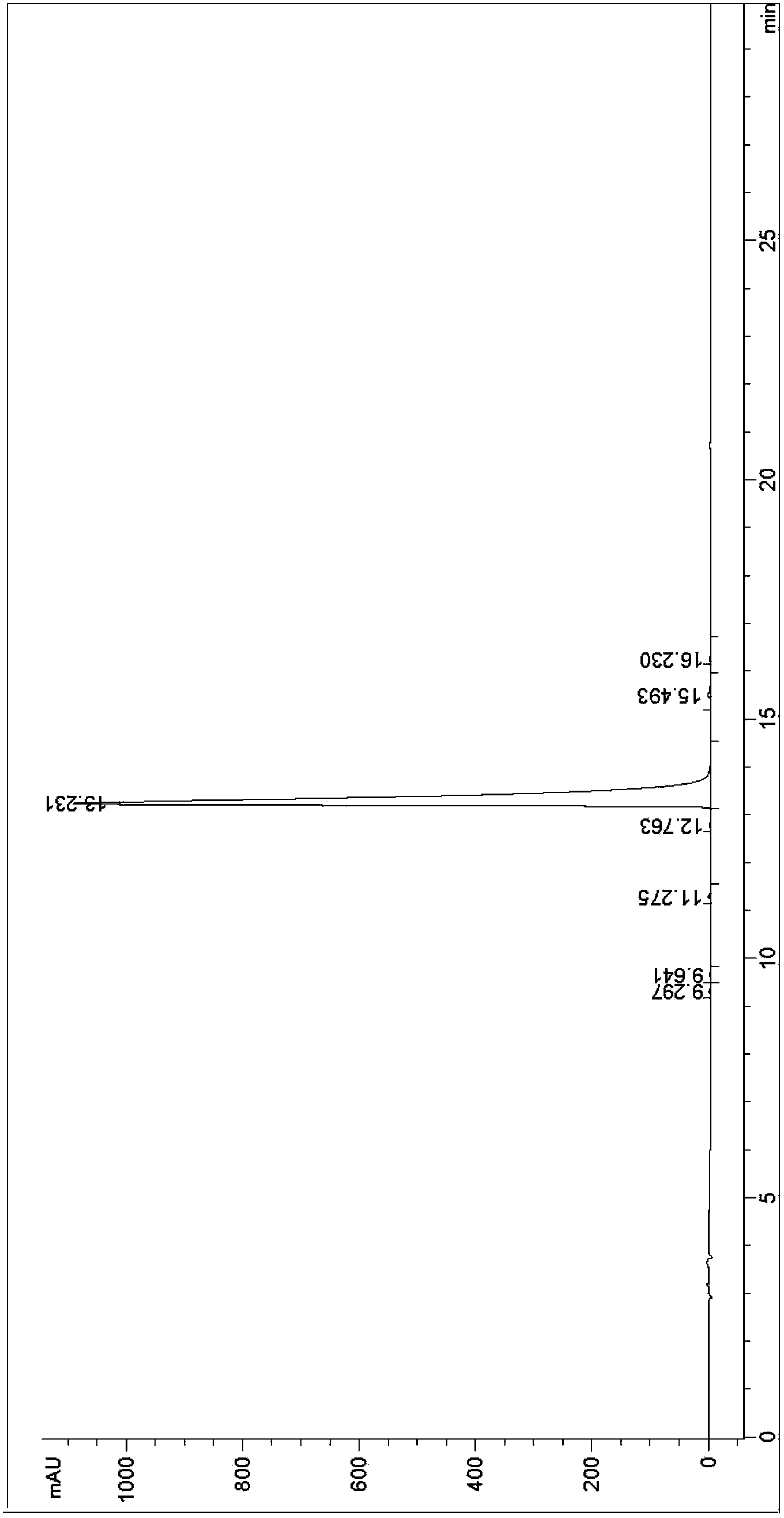
Image
Examples
Embodiment 1
[0040] At room temperature, under nitrogen protection, 2.4g of 1.1-dimethoxy-6-chlorohexane was dissolved in a mixed solution of 24mL of ethanol and 12mL of water, and the temperature was raised to 68°C to completely dissolve. At room temperature, a mixed solvent of 1.9g of 4-cyanophenylhydrazine hydrochloride, 19mL of ethanol and 9.5mL of pure water was slowly added dropwise to the 1.9g of 1.1-dimethoxy-6-chlorohexane reaction solution. Keep warm at 70°C for 0.8 hours, wait for the reaction solution to cool down to room temperature, a large amount of solid precipitates, filter with suction, and recrystallize the filter cake with 50% ethanol aqueous solution to obtain 0.7g of 3-(4-chlorobutyl)-5-cyanoindol Indole.
[0041] At room temperature, add 15mL of anhydrous acetonitrile to the reaction flask under the protection of nitrogen, then add 3.26g of 4-piperazin-1-yl-phenol, 2.35g of MgCl 2 and 6.0mL triethylamine, 1.9g paraformaldehyde, heat up and reflux for 5 hours, wait f...
Embodiment 2
[0045] At room temperature, under nitrogen protection, 5.2 g of 1.1-dimethoxy-6-chlorohexane was dissolved in a mixed solution of 52 mL of methanol and 26 mL of water, and the temperature was raised to 72° C. to completely dissolve. At room temperature, a mixed solvent of 4.8g 4-cyanophenylhydrazine hydrochloride, 48mL methanol and 24mL pure water was slowly added dropwise to the 4.6g1.1-dimethoxy-6-chlorohexane reaction solution, at 72 Keep warm at ℃ for 1.0 hour, wait for the reaction liquid to drop to room temperature, a large amount of solid precipitates, filter with suction, and recrystallize the filter cake with 53% ethanol aqueous solution to obtain 9.5g of 3-(4-chlorobutyl)-5-cyanindole .
[0046] At room temperature, add 50mL tetrahydrofuran to the reaction flask under the protection of nitrogen, then add 3.5g 4-piperazin-1-yl-phenol, 2.4gMgCl 2 and 2.0mL triethylamine, 3.6g paraformaldehyde, heat up and reflux for 4 hours, wait for the reaction solution to cool to r...
Embodiment 3
[0050] At room temperature, under nitrogen protection, 3.6g of 1.1-dimethoxy-6-chlorohexane was dissolved in a mixed solution of 36mL of ethanol and 18mL of water, and the temperature was raised to 72°C to completely dissolve. At room temperature, a mixed solvent of 2.4g 4-cyanophenylhydrazine hydrochloride, 24mL methanol and 12mL pure water was slowly added dropwise to the 4.0g1.1-dimethoxy-6-chlorohexane reaction solution, at 72 Keep warm at ℃ for 1.1 hours, wait until the reaction solution drops to room temperature, a large amount of solid precipitates, filter with suction, and recrystallize the filter cake with 55% ethanol aqueous solution to obtain 4.5g of 3-(4-chlorobutyl)-5-cyanindole .
[0051] At room temperature, add 50mL of toluene to the reaction bottle under the protection of nitrogen, then add 6.0g of 4-piperazin-1-yl-phenol, 3.2g of MgCl 2 And 2.1mL diisopropylethylamine, 3.6g paraformaldehyde, heat up and reflux for 7 hours, wait for the reaction solution to c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com